Novel amino acid self-assembled supermolecular polymer as well as preparation methods and application thereof
A supramolecular polymer and self-assembly technology, applied in botany equipment and methods, detergent compositions, applications, etc., can solve the problems of poor decontamination ability of surfactants, achieve strong cleaning ability, mild reaction conditions, The effect of structural performance stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
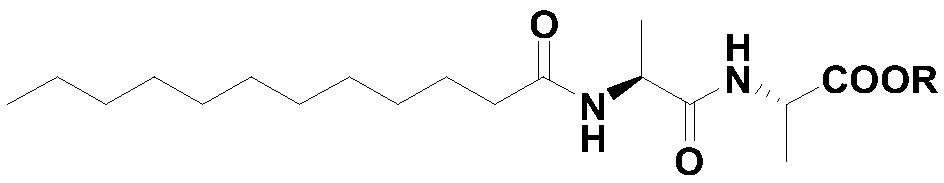
[0100] Example 1 Synthesis of N-lauroyl-L-alanyl-L-alanine self-assembled polymer
example 1
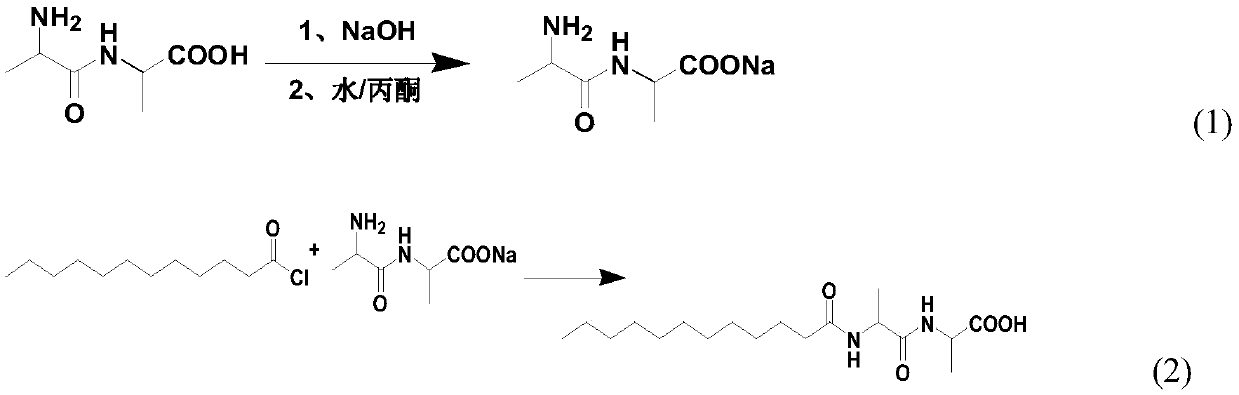
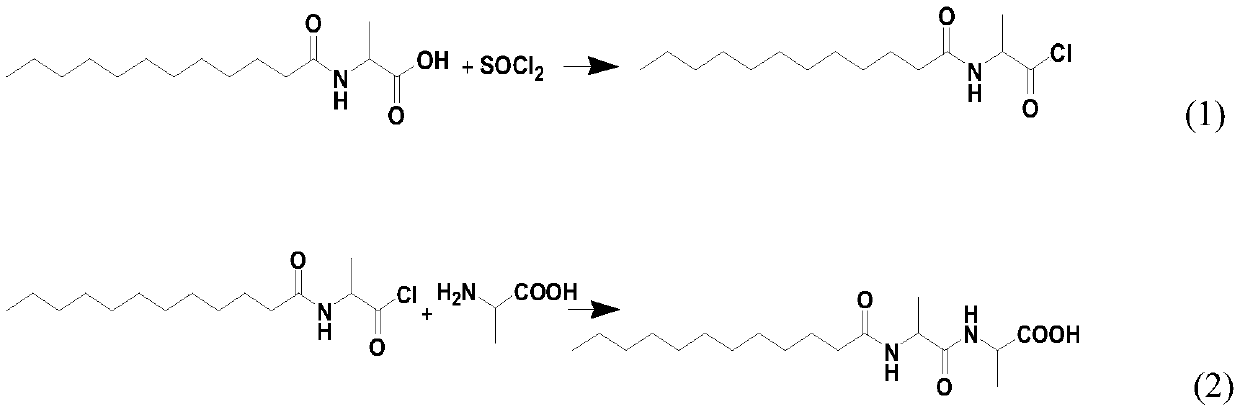
[0102] At room temperature, in a 2L three-neck flask, dissolve 16.0g (0.1mol) L-alanyl-L-alanine and 4..0g (0.1mol) sodium hydroxide in a mixed solution of 450mL distilled water and 450mL acetone Stir in medium to obtain L-alanyl-L-alanine sodium solution.
[0103] Under the condition of 25 DEG C, in the L-alanyl-L-alanine salt solution, slowly add 21.9g (0.1mol) lauroyl chloride dropwise, then add dropwise 50% sodium hydroxide solution so that the pH of the reaction system= 9. After the dropwise addition, continue stirring at 25° C. for 2 h to obtain pasty N-lauroyl-L-alanyl-L-alanine salt.
[0104] Add hydrochloric acid to the pasty N-lauroyl-L-alanyl-L-alanine salt to acidify to pH = 3~4, gradually precipitate a white solid, then place it in an ice bath for 2 hours and filter to obtain N- Lauroyl-L-alanyl-L-alanine crude.
[0105] Add a mixed solvent of water and acetone, L-alanyl-L-alanine and p-toluenesulfonic acid to the crude N-lauroyl-L-alanyl-L-alanine, wherein, N-l...
example 2
[0108] At room temperature, in a 2L three-neck flask, dissolve 16.0g (0.1mol) L-alanyl-L-alanine and 6.0g (0.15mol) potassium hydroxide in a mixed solution of 450mL distilled water and 450mL acetonitrile and stir Uniformly obtain L-alanyl-L-alanine sodium solution.
[0109] Under the condition of 25 DEG C, in the L-alanyl-L-alanine salt solution, slowly add 17.52g (0.08mol) lauroyl chloride dropwise, then add dropwise 30% sodium hydroxide solution so that the pH of the reaction system= 9. After the dropwise addition, continue stirring at 50° C. for 0.5 h to obtain pasty N-lauroyl-L-alanyl-L-alanine salt.
[0110] Add hydrochloric acid to the pasty N-lauroyl-L-alanyl-L-alanine salt to acidify to pH = 3~4, gradually precipitate a white solid, then place it in an ice bath for 3 hours and filter to obtain N- Lauroyl-L-alanyl-L-alanine crude.
[0111] Add a mixed solvent of water and acetone, L-alanyl-L-alanine and p-toluenesulfonic acid to the crude N-lauroyl-L-alanyl-L-alanine,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com