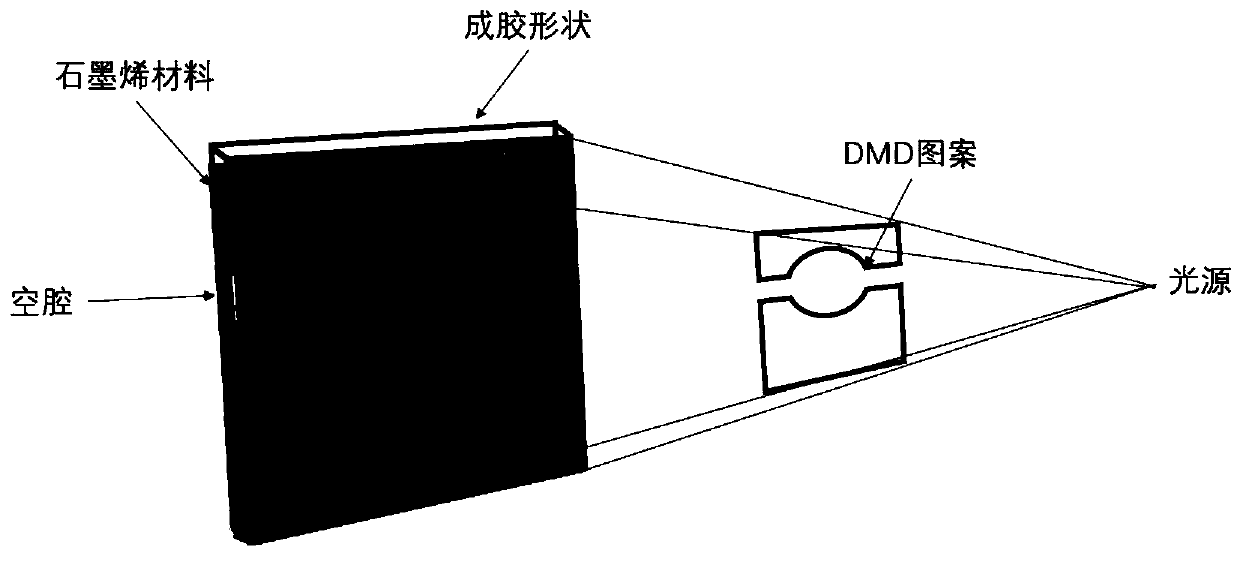
Three-dimensional bubble graphene-PEGDA-GelMA photocuring biological material for loading cells, and preparation method and application thereof
A biomaterial and light-curing technology, applied in biochemical equipment and methods, immobilized enzymes, immobilized on or in inorganic carriers, etc., can solve 3DGF structure fragility, insufficient biocompatibility, insufficient mechanical strength, etc. problem, to achieve good biocompatibility, good electrical conductivity, high mechanical strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] In this example, taking 10% PEGDA mixed with 10% GelMA loaded mouse microglial cell line BV2 cells as an example, the medium uses DMEM high glucose + 10% FBS cell culture medium to configure and prepare three-dimensional vesicles loaded with cells Graphene-PEGDA-GelMA photocurable biomaterials.
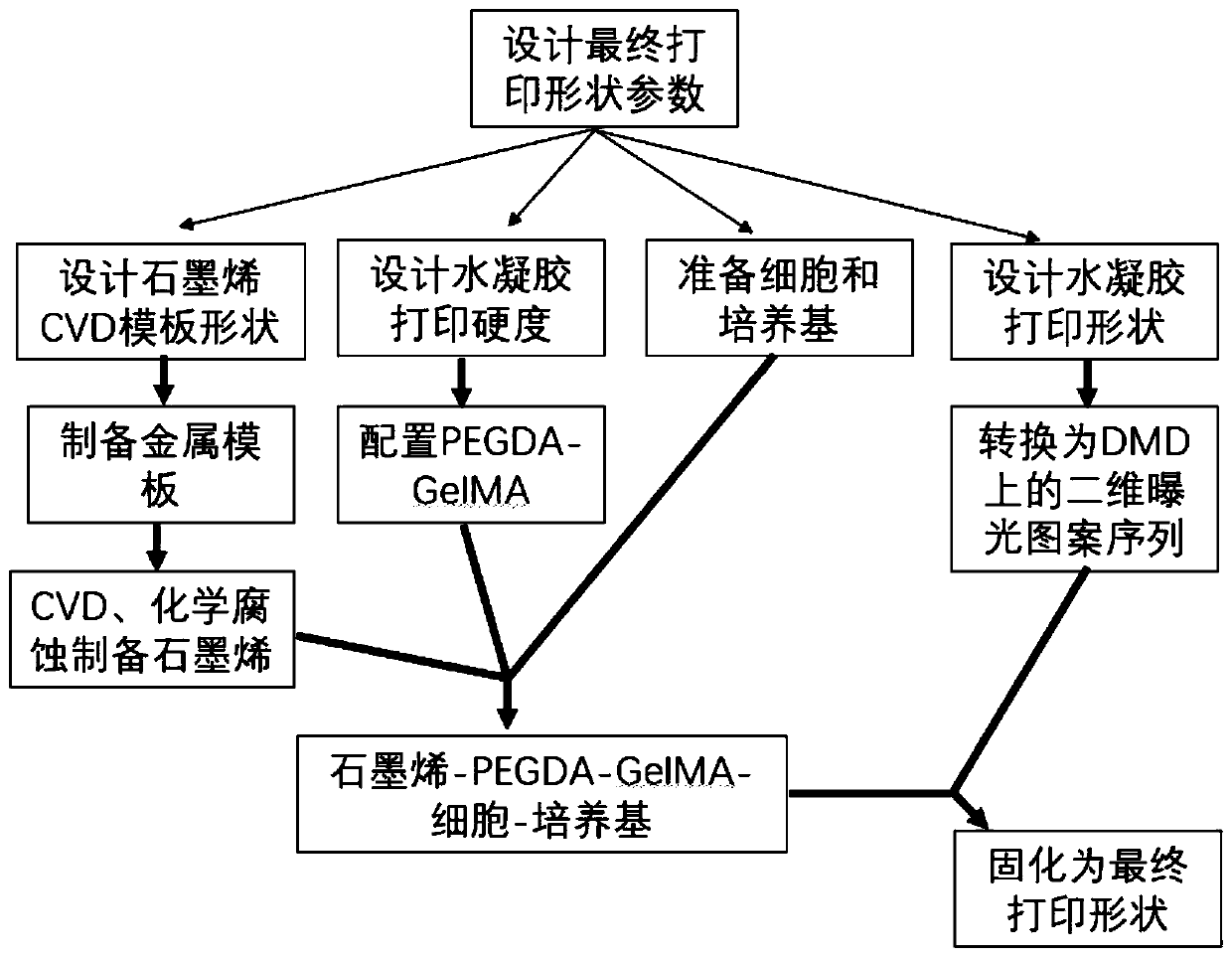
[0069] Its design and preparation process is as follows figure 1 As shown, it mainly includes designing the final printing shape parameters first, including designing the shape of graphene CVD template, designing the hardness of hydrogel printing, preparing cells and culture medium, and printing shape involving hydrogel. By preparing a metal template according to the shape of the graphene CVD template, a graphene material (that is, three-dimensional bubble graphene) is prepared by chemical vapor deposition (CVD) or chemical corrosion; according to the designed hydrogel printing hardness, the configuration contains PEGDA and The mixture of GelMA, and then the mixture of PEGDA a...
Embodiment 2
[0076] In this example, 10% PEGDA mixed with 10% GelMA loaded human umbilical vein endothelial cells HUVEC cells is taken as an example, and the medium uses EGM2 medium to configure and prepare a three-dimensional cell-loaded graphene-PEGDA-GelMA light-cured biomaterial , whose design and preparation process is as follows figure 1 shown.
[0077] Its preparation method is as follows:
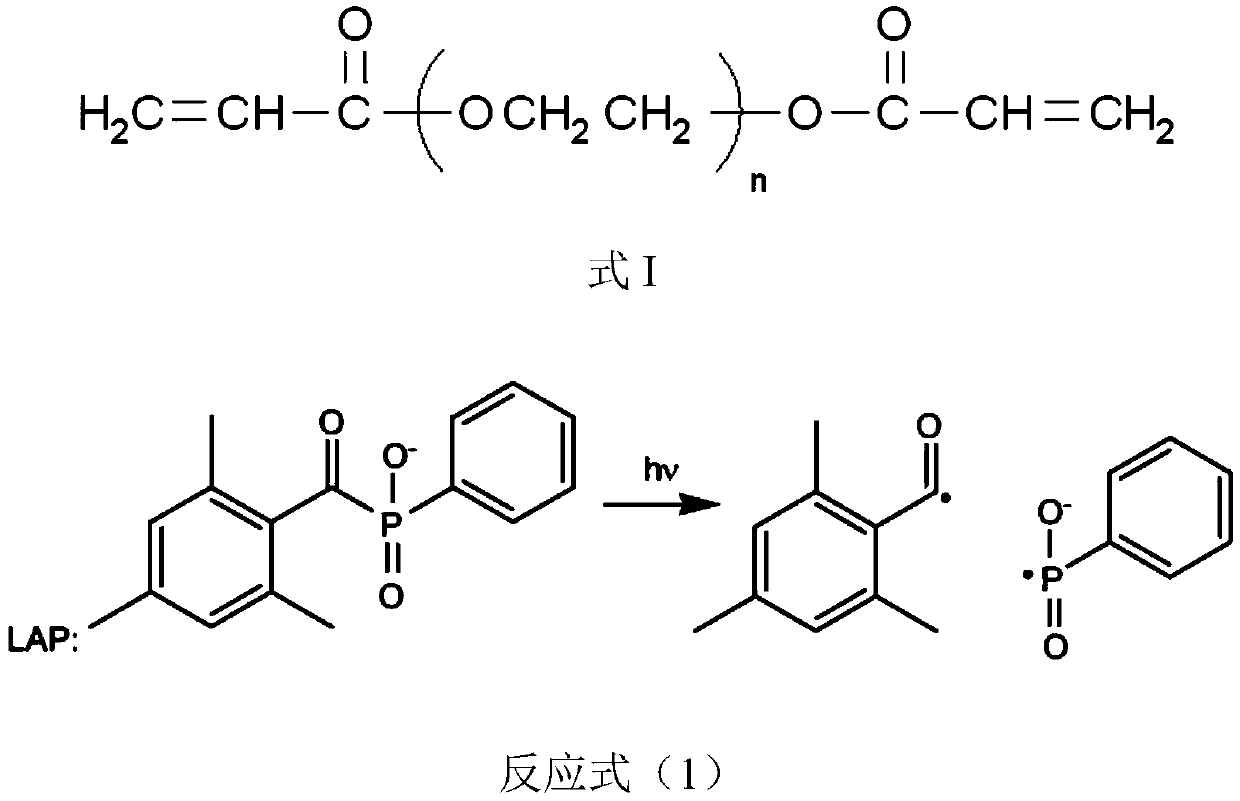
[0078] (1) Take out the sterilized and sterile PEGDA and GelMA stored in the refrigerator in advance, and let it stand at room temperature until the PEGDA is completely melted into a liquid; configure cell culture medium (EGM2 medium, filter-sterilized); weigh 0.3g LAP , 15g PEGDA-700 and 10g GelMA, mixed in 100mL of cell culture medium (hydrogel medium mixture) in a sterile environment, heated in a metal bath at 37°C to melt and mix the GelMA evenly to obtain a hydrogel medium mixture , and stored at 37°C.
[0079] (2) To prepare HUVEC cells for 3D printing, add the suspended cells to the hy...
Embodiment 3
[0083] In this example, 10% PEGDA mixed with 10% GelMA loaded human cervical cancer cell line HeLa cells is taken as an example, and the medium uses DMEM+10% FBS cell culture medium to configure and prepare three-dimensional vesicular graphene-PEGDA loaded with cells -GelMA photocurable biomaterial, its design and preparation process is as follows figure 1 shown.
[0084] Its preparation method is as follows:
[0085] (1) Take out the sterilized and preserved sterile PEGDA and GelMA from the refrigerator in advance, and let it stand at room temperature until the PEGDA is completely melted into a liquid; configure cell culture medium (DMEM+10% FBS, sterilized by filtration); weigh 0.5 g LAP, 10 g PEGDA-700, and 15 g GelMA were mixed in 100 mL of cell culture medium (hydrogel medium mixture) in a sterile environment, heated in a 37°C water bath to melt and mix the GelMA evenly, and stored at 37°C.
[0086] (2) To prepare HeLa cells for 3D printing, add the suspended cells to t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com