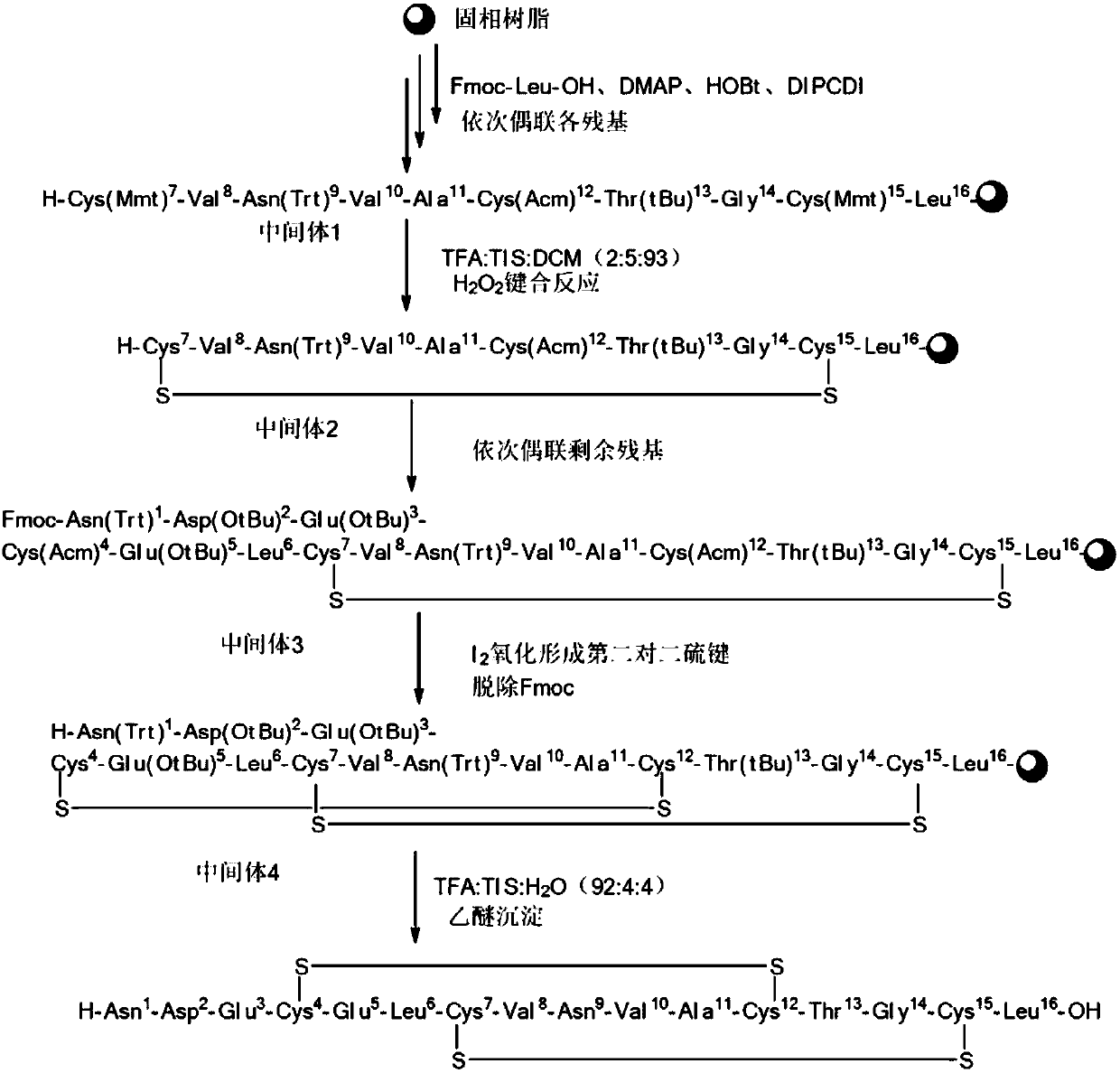
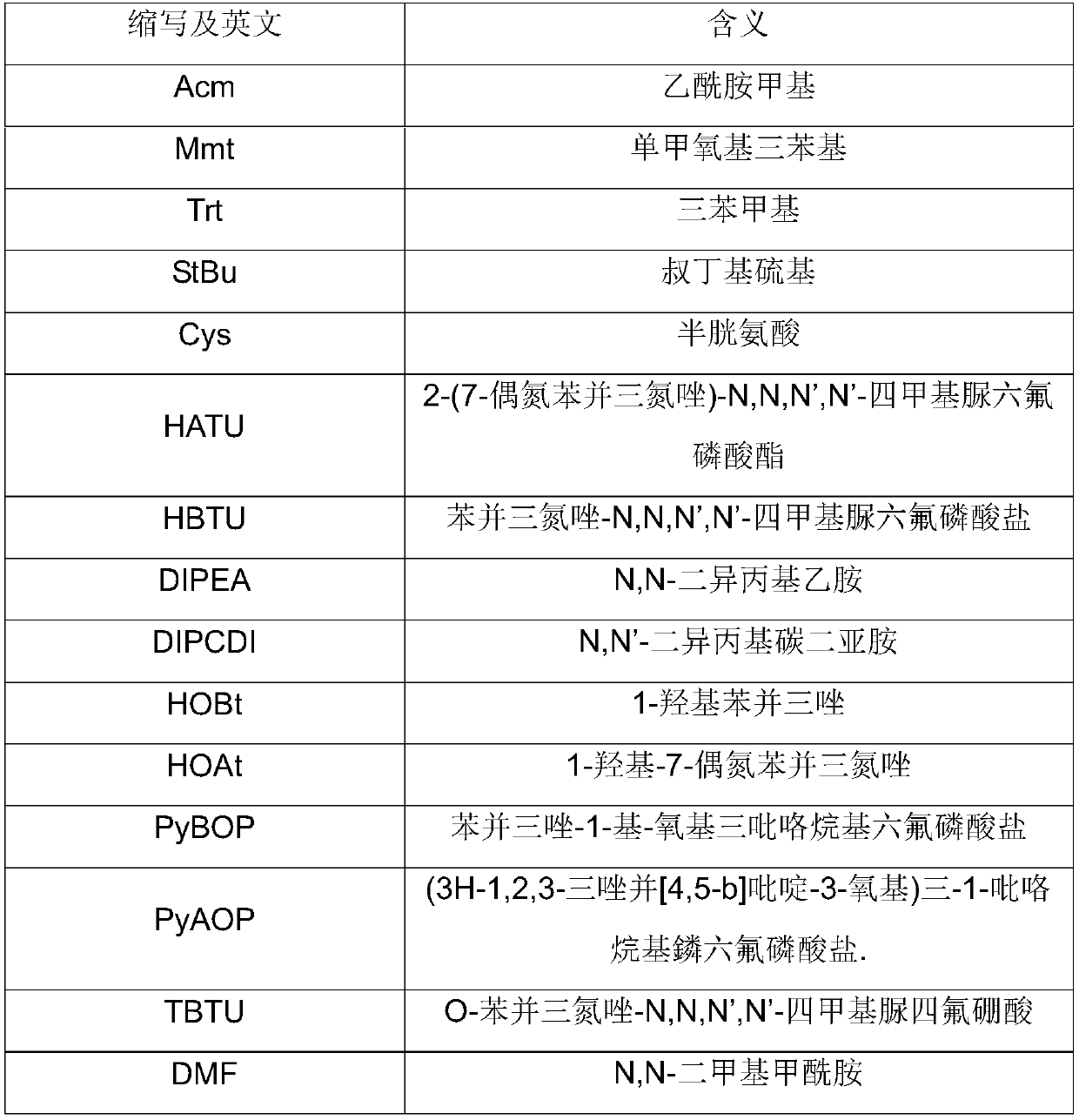
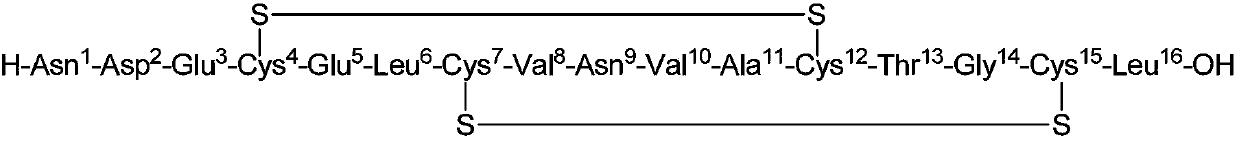Preparation method of plecanatide
A technology of plecanatide and -OH, applied in the field of peptide synthesis, can solve the problems of severe resin shrinkage, low yield of final product, difficult coupling, etc., and achieve the effect of reducing coupling difficulty, simple and feasible operation, and improving purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Preparation of Fmoc-Leu-Wang Resin
[0044] Weigh 150g of Wang resin with a degree of substitution of 1.03mmol / g in a solid-phase reaction column, wash it twice with DMF and swell the resin with DMF for 30 minutes, get 37.5g Fmoc-Leu-OH, 15.6g HOBt, and 1.29gDMAP in 400ml of a mixed solution of DCM and DMF with a volume ratio of 1:1 was stirred in an ice-salt water bath. When the temperature was controlled at 0-10°C, 17.4g of DIPCDI was added dropwise and activated for 2-3 minutes. The above solution was added to a solid-phase reaction column and reacted at room temperature for 1.5 h. After the reaction was finished, wash with DMF for 4 times, then add 140 mL of acetic anhydride, 120 mL of pyridine and an appropriate amount of DMF, and mix and seal the reaction for more than 8 hours. After the reaction, wash 4 times with DMF and 2 times with DCM. After shrinking by methanol twice, the resin was drained to obtain 160.5 g of Fmoc-Leu-Wang resin, and the de...
Embodiment 2
[0045] Embodiment 2: the preparation of peptide resin
[0046] The Fmoc-Leu-Wang resin 47.6g (10mmol) of 0.21mmol / g degree of substitution prepared by weighing Example 1 is placed in a solid-phase reaction column, washed with DMF for 2 times and then used to swell the resin with DMF for 30 minutes, and then use 150ml of DBLK was deprotected for 5min+7min, washed 6 times with 150mL DMF.
[0047] Weigh 30.8g (50mmol) Fmoc-Cys(Mmt)-OH and 8.1g (60mmol) HOBt dissolved in 100mL of DMF / DCM (1:1), add 10mL (65mmol) DIPCDI under ice-water bath for 2-3min activation, Add the mixed solution into the reaction column, react at room temperature for 2 hours, and detect the end of the reaction with ninhydrin (if the resin is colorless and transparent, stop the reaction; if the resin develops color, prolong the reaction for 1 hour). After the reaction is over, wash the resin with 150mL DMF for 3 times, add 150mL DBLK for deprotection for 6min + 8min, wash the resin with 150mLDMF for 6 times,...
Embodiment 3
[0053] Example 3: Cleavage of Peptide Resins
[0054] The 78.6g (10mmol) peptide resin (1) obtained in Example 2 is all placed in the cleavage reactor, and the cleavage reagent (TFA:TIS:H 2 O=92:4:4 (V / V)), stirred at room temperature for 2h. The reactant was filtered with a sand core funnel, after the filtration was completed, a small amount of TFA was added to wash the resin, the filtrate was collected and combined, and concentrated under reduced pressure to a certain volume. Add frozen anhydrous ether (100ml / g peptide resin) to precipitate the solution, centrifuge, remove the supernatant, wash the precipitate three times with anhydrous ether, and vacuum dry to obtain 17.2g of crude peptide as a white solid powder. The purity of the crude peptide is 81.3% (HPLC testing conditions: Waters C18 300A 1.7um 2.0*100mm; buffer: 50mM ammonium phosphate buffer (phosphoric acid adjusted pH=6.2); phase A: acetonitrile: buffer = 10:90 (V: V); Phase B: buffer solution = 50:50 (V:V), gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com