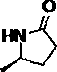
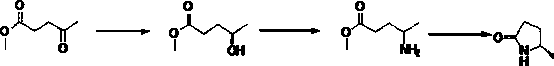Synthesis method of (R)-5-methylpyrrolidone-2-one
A technology of methylpyrrolidone and synthetic method, which is applied in the direction of organic chemistry, organic chemistry, etc., can solve the problems of expensive raw materials, poor universality, and high development difficulty, and achieve high yield, low raw material price, and atom utilization high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
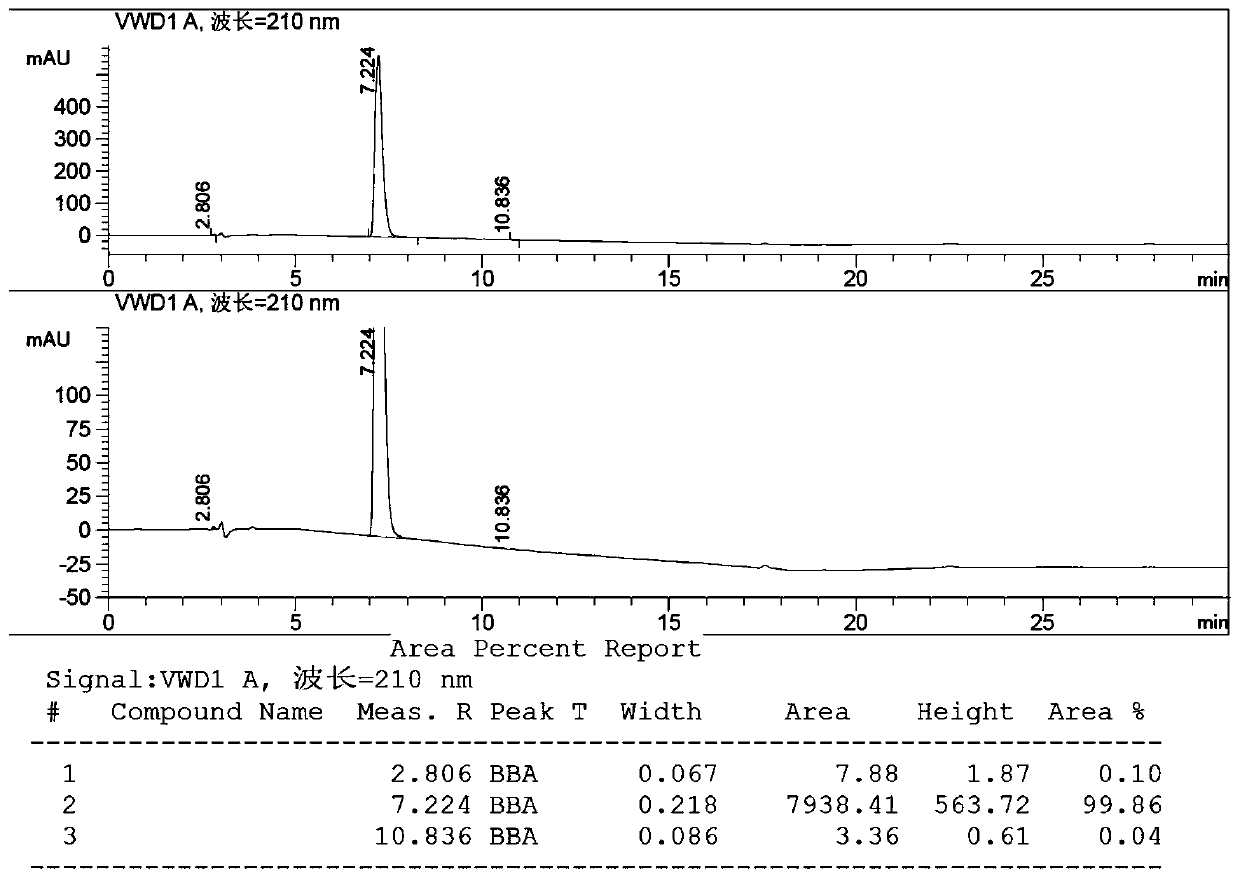
[0033] Example 1: In a 1L three-necked flask, add 500 mL of dichloromethane, cool down by about 5 degrees in an ice bath, add 80 g of methyl levulinate, 17 g of (S)-Me-CBS, pass nitrogen flow, and dropwise add borane dimethyl Thioether 56.04g, after dropping and stirring for 0.5h, the temperature was raised to 30°C and reacted for 3h, and the reaction was completed as detected by HPLC. Methanol was added dropwise, hydrochloric acid was used to quench the reaction, and 69.8 g of (R)-4-hydroxyvalerate methyl ester was obtained after treatment, >99% ee, yield 85.8%.
[0034] (R) 50g of methyl 4-hydroxyvalerate was put into a 2L autoclave, 400g of ammonia water, 400g of methanol, and 5g of Raney nickel were added. The temperature was increased to 90°C, and the reaction was completed in 6h. The catalyst was filtered, water and EA were added for extraction, and 48 g of (R)-4-aminovaleric acid methyl ester was obtained after the treatment, with a yield of 96.8%.
[0035] (R) 50 g o...
Embodiment 2
[0036] Example 2: In a 1L three-necked flask, add 500 mL of acetonitrile, lower the temperature in an ice bath by about 5 degrees, add 80 g of methyl levulinate, 17 g of (S)-Me-CBS, pass nitrogen flow, and add borane dimethyl sulfide dropwise 56.04g, stirred for 0.5h after dropping, heated to 30°C and reacted for 3h, and HPLC detected the end of the reaction. Methanol was added dropwise, hydrochloric acid was used to quench the reaction, and 70.5 g of (R)-4-hydroxyvalerate methyl ester was obtained after treatment, >99% ee, yield 86.6%.
[0037] (R) 50g of methyl 4-hydroxyvalerate was put into a 2L autoclave, 400g of ammonia water, 400g of methanol, and 5g of Raney nickel were added. The temperature was increased to 90°C, and the reaction was completed in 6h. The catalyst was filtered, water and EA were added for extraction, and 47 g of (R)-4-aminovaleric acid methyl ester was obtained after the treatment, with a yield of 94.8%.
[0038] (R) 50g of methyl 4-aminovalerate was...
Embodiment 3
[0039]Example 3: In a 1L three-necked flask, add 500 mL of dimethylformamide, lower the temperature in an ice bath by about 5 degrees, add 80 g of methyl levulinate and 17 g of (S)-Me-CBS, pass nitrogen flow, and add borane dropwise 56.04 g of dimethyl sulfide, stirred for 0.5 h after dropping, heated to 30° C. and reacted for 3 h, and the reaction was completed as detected by HPLC. Methanol was added dropwise, hydrochloric acid was used to quench the reaction, and 70 g of (R)-4-hydroxyvalerate methyl ester was obtained after treatment, >99% ee, yield 86%.
[0040] (R) 50g of methyl 4-hydroxyvalerate was put into a 2L autoclave, 400g of ammonia water, 400g of methanol, and 5g of Raney nickel were added. The temperature was increased to 90°C, and the reaction was completed in 6h. The catalyst was filtered, water and EA were added for extraction, and 49 g of (R)-4-aminovaleric acid methyl ester was obtained after the treatment, with a yield of 98.8%.
[0041] (R) 50 g of methy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com