Organic compound and application thereof
An organic compound, selected technology, applied in the direction of organic chemistry, compounds of Group 5/15 elements of the periodic table, chemical instruments and methods, etc., can solve the problem that the photoelectric performance cannot meet the requirements of high-performance OLED devices, etc., to achieve improved luminescence Effects of brightness and external quantum efficiency, reduced efficiency roll-off, and broadened exciton recombination region
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
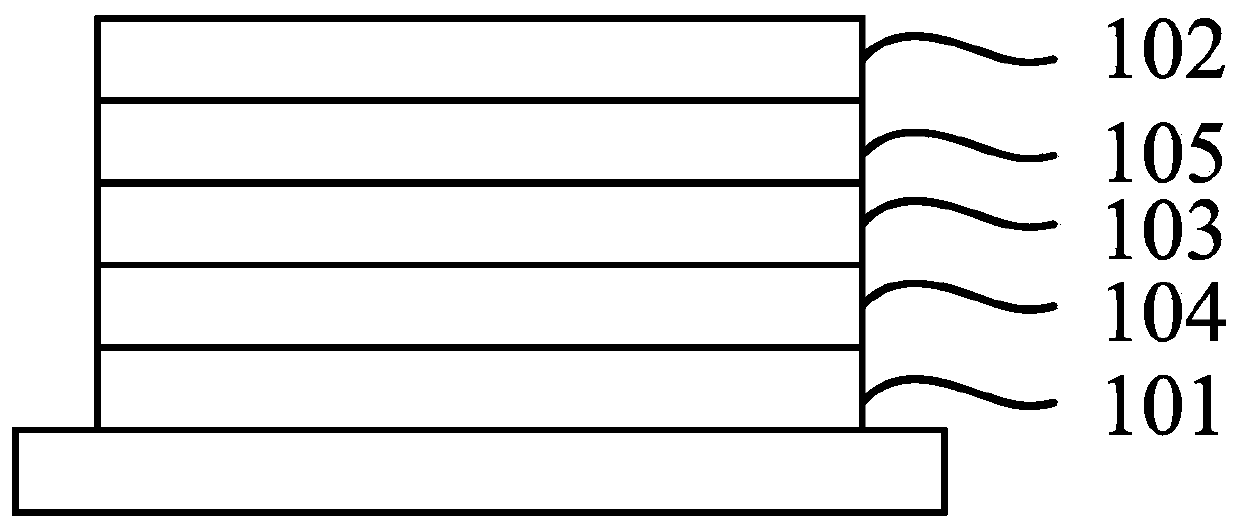
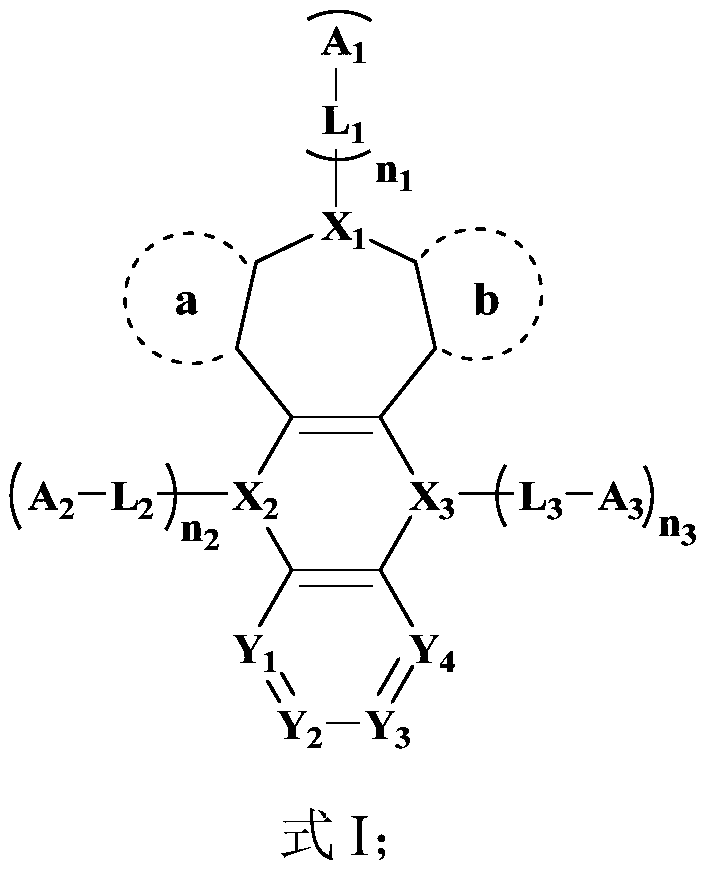
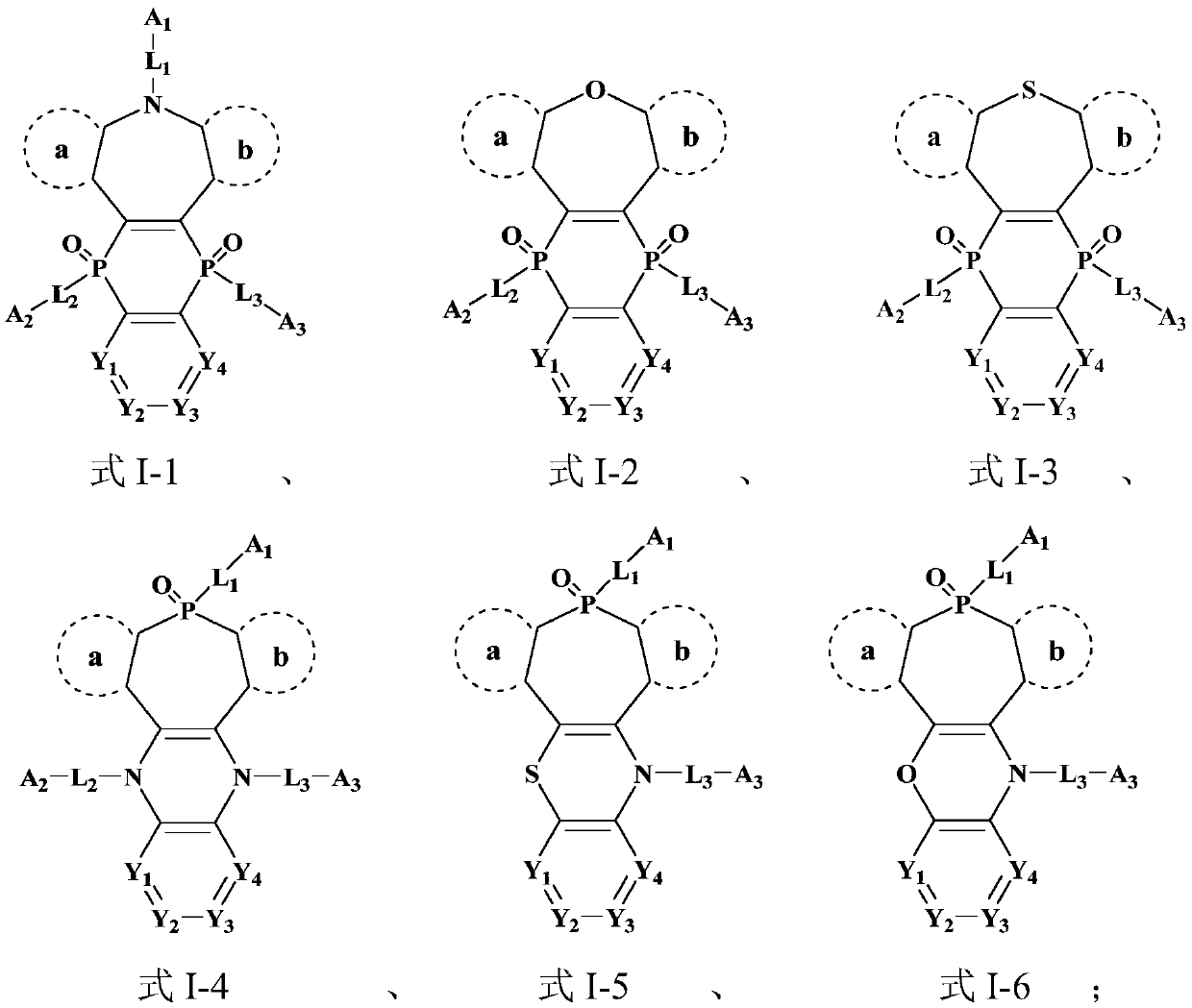
Image
Examples
Embodiment 1
[0081] This embodiment provides an organic compound with the following structure:
[0082]
[0083] The synthetic route is as follows:
[0084]
[0085] Concrete preparation method specifically comprises the following steps:
[0086] (1) In a 250mL round bottom flask, the raw material M2-1 (15mmol) and Br 2 (35mmol) was added into dry carbon tetrachloride (100mL), and reacted at room temperature under a nitrogen atmosphere for 24h; the obtained intermediate mixed solution was added to water, then filtered through a diatomaceous earth pad, and the filtrate was extracted with dichloromethane, After washing with water and drying over anhydrous magnesium sulfate, after filtration and evaporation, the crude product was purified by silica gel column chromatography to obtain intermediate M2-2.
[0087] (2) In a 250mL round bottom flask, intermediate M2-2 (15mmol) and 1,2-bis(diphenylphosphine)ethane nickel chloride (NidppeCl 2 , 10%, 1.5mmol) was added into dry tetrahydrofur...
Embodiment 2
[0094] This embodiment provides an organic compound with the following structure:
[0095]
[0096] The synthetic route is as follows:
[0097]
[0098] Concrete preparation method specifically comprises the following steps:
[0099] (1) 5H dibenzo[b,f]azepine (10mmol), 2-bromodiphenylfuran (12mmol), tridibenzylideneacetone dipalladium Pd 2 (dba) 3 (0.05mmol), sodium tert-butoxide (14mmol), tri-tert-butylphosphorus P(t-Bu) 3 (0.2mmol) was put into a 50mL three-necked flask, and the degassing and nitrogen replacement were repeated 3 times rapidly while stirring, and 20mL of toluene was added through a syringe; the mixture was heated to reflux under nitrogen flow for 3h; Add water to the mixture, extract with dichloromethane, wash with saturated brine, dry the organic layer with anhydrous sodium sulfate, distill off the solvent, and use column chromatography for purification to obtain intermediate M12-1 .
[0100] (2) In a 250mL round bottom flask, intermediate M12-1 ...
Embodiment 3
[0108] This embodiment provides an organic compound with the following structure:
[0109]
[0110] The synthetic route is as follows:
[0111]
[0112] Concrete preparation method specifically comprises the following steps:
[0113] (1) 1,4-dihydroquinoxaline (10mmol), 1-bromonaphthalene (24mmol), tridibenzylideneacetone dipalladium Pd 2 (dba) 3 (0.05mmol), sodium tert-butoxide (14mmol), tri-tert-butylphosphorus P(t-Bu) 3 (0.2mmol) was put into a 50mL three-necked flask, and the degassing and nitrogen replacement were repeated 3 times rapidly while stirring, and 20mL of toluene was added through a syringe; the mixture was heated to reflux under nitrogen flow for 3h; Add water to the mixture, extract with dichloromethane, wash with saturated brine, dry the organic layer with anhydrous sodium sulfate, distill off the solvent, and use column chromatography for purification to obtain intermediate M36-1 .
[0114] (2) In a 250mL round bottom flask, intermediate M36-1 (1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com