Detection method for 1-(2, 3-dichlorophenyl) piperazine hydrochloride and related substances thereof
A detection method, the technology of dichlorophenyl, applied in the field of detection, can solve the problems of affecting the purity of aripiprazole, the inability to detect effectively, the increase of related substances, etc., and achieve high sensitivity, short peak time and good separation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
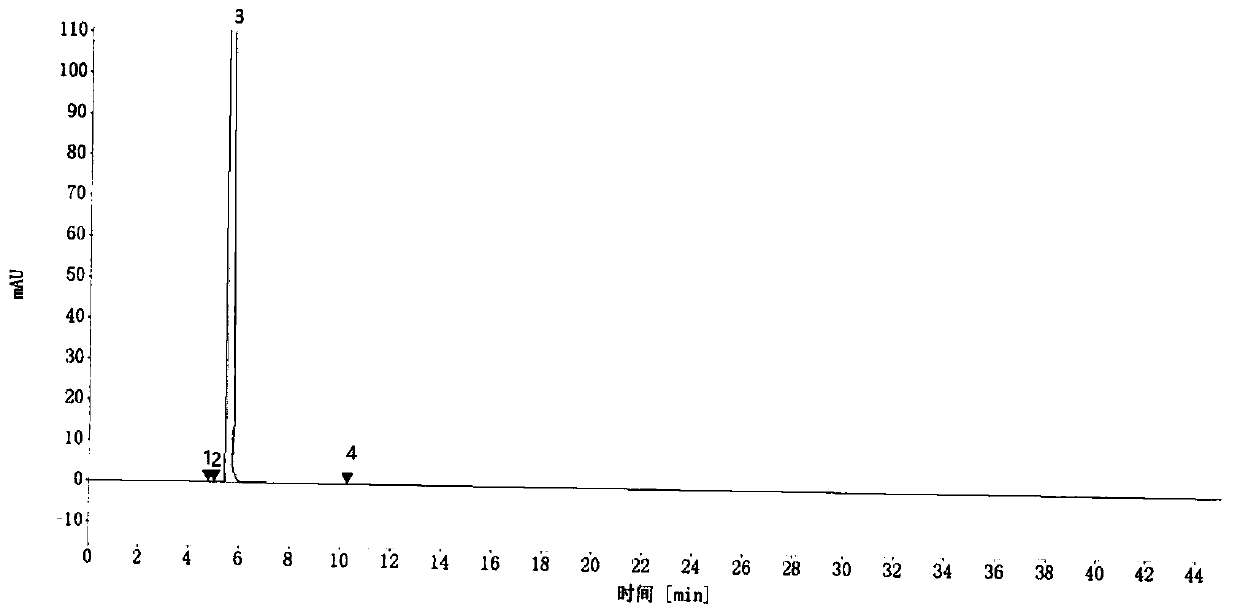
Embodiment 1
[0122] 1. Sample preparation
[0123] Preparation of sample solution: take an appropriate amount of 1-(2,3-dichlorophenyl)piperazine hydrochloride sample, add acetonitrile to make a 0.4mg / ml solution of the test solution;
[0124] Preparation of reference solution: take 2,3-dichloroaniline, 1-(2-chlorophenyl)piperazine hydrochloride, 1-(3-chlorophenyl)piperazine hydrochloride, 1-(2,3 -Chlorophenyl)piperazine dimer hydrochloric acid reference substance in appropriate amount, accurately weighed, add acetonitrile to make 0.4μg of 2,3-dichloroaniline, 1-(2-chlorophenyl)piperazine hydrochloride per 1ml A mixed solution of 0.4 μg, 0.4 μg of 1-(3-chlorophenyl)piperazine hydrochloride, and 0.4 μg of 1-(2,3-chlorophenyl)piperazine dimer hydrochloride is obtained;
[0125] 2. Detection
[0126] time (min) Mobile phase A (%) Mobile phase B (%) 0 85 15 10 70 30 25 15 85 25.1 85 15 30 85 15
[0127] name Quantitation limit (ug / m...
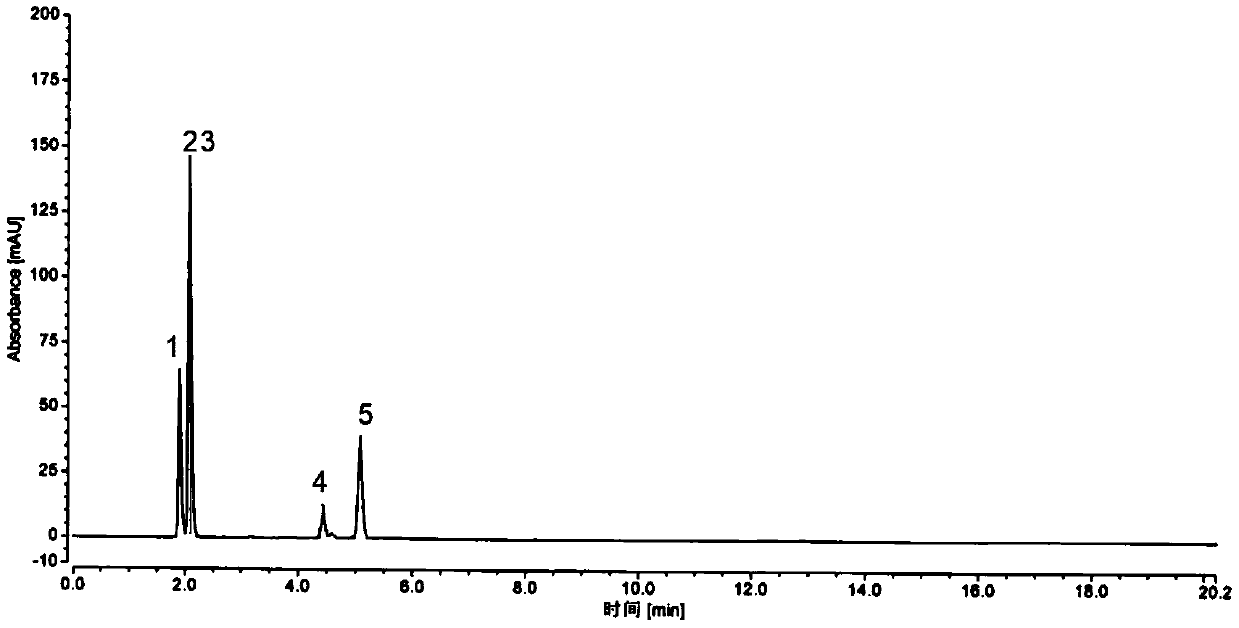
Embodiment 2
[0129] 1. Sample preparation
[0130] Test sample solution preparation: Example 1
[0131] Preparation of reference solution: Example 1
[0132] 2. Detection
[0133] time (min) Mobile phase A (%) Mobile phase B (%) 0 85 15 10 70 30 25 15 85 25.1 85 15 30 85 15
[0134] name Quantitation limit (ug / ml) Detection limit (ug / ml) degree of separation Impurity 1 0.04 0.01 6.46 14954 Impurity 2 0.01ug / ml 0.003ug / ml 7.43 27558 main ingredient 0.04ug / ml 0.013ug / ml 21.53 5529 Impurity 3 0.02ug / ml 0.005ug / ml 23.73 378133 Impurity 4 0.04ug / ml 0.01ug / ml —— 83449
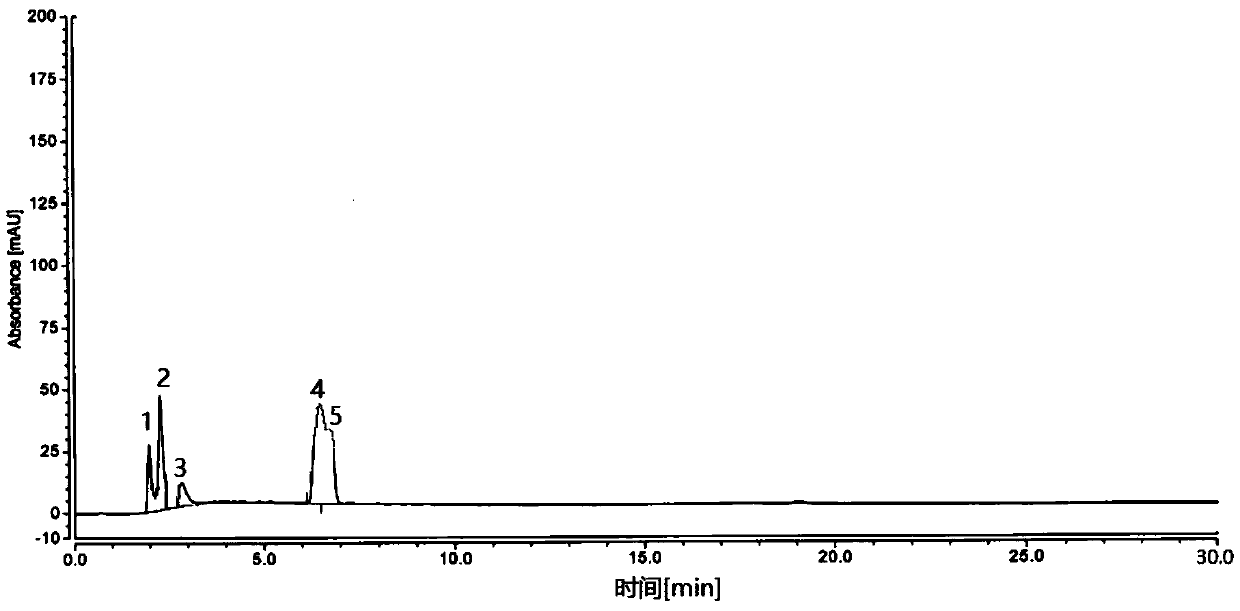
Embodiment 3
[0136] 1. Sample preparation
[0137] Test sample solution preparation: Example 1
[0138] Preparation of reference solution: Example 1
[0139] 2. Detection
[0140] time (min) Mobile phase A (%) Mobile phase B (%) 0 85 15 10 70 30 25 15 85 25.1 85 15 30 85 15
[0141] name Quantitation limit (ug / ml) Detection limit (ug / ml) degree of separation Number of theoretical plates Impurity 1 0.04ug / ml 0.01ug / ml 6.49 15422 Impurity 2 0.01ug / ml 0.003ug / ml 7.35 29029 main ingredient 0.04ug / ml 0.013ug / ml 20.28 5739 Impurity 3 0.03ug / ml 0.01ug / ml 9.25 395840 Impurity 4 0.04ug / ml 0.01ug / ml —— 26697
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com