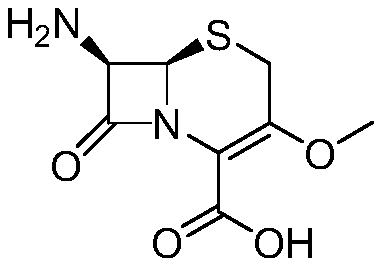
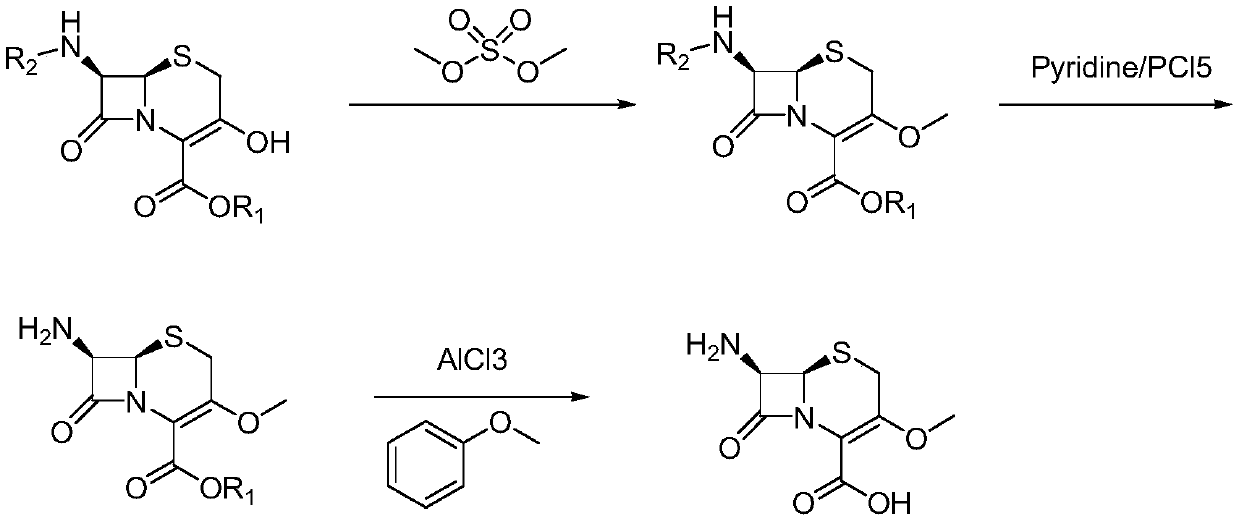
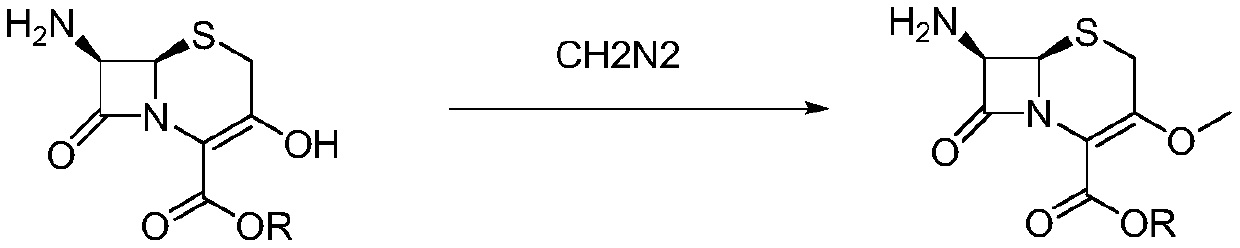
Novel method for preparing cefroxadine parent nucleus 7-AMOCA
A technology of cefoxadin and a new method, which is applied in the field of preparation of cephalosporin intermediates, can solve the problems of easy explosion, strong stimulation, and dyspnea, and achieve the goals of improving production safety, mild process operating conditions, and reducing costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The present invention proposes a kind of novel method for preparing cefoxadine mother nucleus 7-AMOCA, comprises the steps:
[0030] (1) Preparation of methides:
[0031] Take a 1000ml dry three-necked flask, add 50g (0.099mol) of 3-hydroxycephalosporin, 300ml of N, N-dimethylformamide, 22g (0.1584mol) of potassium carbonate under mechanical stirring; heat up to 60-65°C after stirring well ; then dropwise add 53.99g (0.594mol) of dimethyl carbonate, and stir for 3 hours after the dropwise addition is completed for 30 minutes; after the reaction is completed, cool down to 25°C; pour it into 500ml of water and 300ml of dichloromethane, stir well , standing and layering; the water phase was extracted with 130ml of dichloromethane, the dichloromethane layers were combined, dried over anhydrous magnesium sulfate for 2 hours, the desiccant was filtered off, and the dichloromethane was recovered to the end to obtain a white methylated solid 46.1 g (0.090 mol), yield 89.7%.
...
Embodiment 2
[0035] The present invention proposes a kind of novel method for preparing cefoxadine mother nucleus 7-AMOCA, comprises the steps:
[0036] (1) Preparation of reduced product:
[0037] Take a 1000ml dry three-neck flask, add 62g (0.124mol) of 3-hydroxycephalosporin, 350ml of N, N-dimethylformamide, 27.35g (0.198mol) of potassium carbonate under mechanical stirring; heat up to 60-65 ℃; then dropwise add 111.58g (1.239mol) of dimethyl carbonate, and stir for 3 hours after the dropwise addition is completed for 30 minutes; after the reaction is completed, cool down to 25°C; pour it into 600ml of water and 320ml of dichloromethane, and stir evenly After standing, the layers were separated; the water phase was extracted with 150ml of dichloromethane, the dichloromethane layers were combined, dried over anhydrous magnesium sulfate for 2 hours, the desiccant was filtered off, and the dichloromethane was recovered to the end to obtain a white methylated solid 60.1 g (0.117 mol), yiel...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com