Clinical application-grade adipose-derived stem cells and preparation method thereof
An adipose stem cell, clinical application technology, applied in cell dissociation methods, biochemical equipment and methods, applications, etc., can solve the problems of low cell survival rate, low yield of nucleated cells, long separation and preparation time, etc. short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Preparation of Adipose Stem Cells
[0071] 1. Preparation of collection bottles
[0072] A fat sample preservation solution was prepared, and the components of the preservation solution included: 40 ml of DMEM, 0.8 ml of penicillin and streptomycin (containing 10,000 IU / ml of penicillin and 10,000 μg / ml of streptomycin; Gibco). Add the prepared preservation solution into the collection bottle, marked as A. Add the same 40ml volume of normal saline to the collection bottle, labeled B.
[0073] 2. Screening of fat samples
[0074] The requirements for fat samples are as follows:
[0075] 1. Hepatitis B, hepatitis C, syphilis, AIDS, CMV, EBV, HTLV virus tests are negative,
[0076] Its detection method is a routine detection method in the art.
[0077] 2. The donor is in good physical condition, without genetic family history, malignant tumor, autoimmune disease, acute or chronic infectious disease, congenital disease, blood system disease or other diseases ...
Embodiment 2
[0103] Embodiment 2 Digestive fluid of the present invention different component distribution ratio and culture effect (weight percentage)
[0104]
[0105] Table 1: The proportion and effect data of the components used in the digestive juice
Embodiment 3
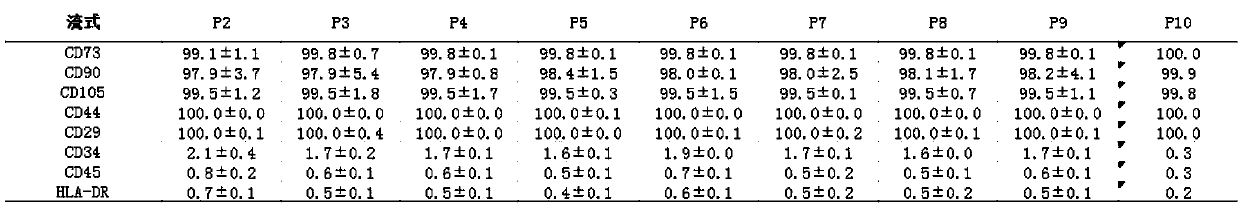
[0106] Note: The unit of cell number in Table 2 is ×10 5 NC / mL Fat Example 3, Detection of Adipose Stem Cells
[0107] 1. Observation of cell morphology
[0108] Adherent cells can usually be seen separating out from the adipose stem cells on the third day, and the cells can reach 80% confluence on the 7th to 10th day. After subculture, the cells were evenly distributed and grew into a spindle. Such as figure 1 shown.
[0109] 2. Detection of nucleated cells per milliliter of adipose tissue
[0110] The number of nucleated cells obtained from the adipose tissue preserved in the preservation solution was: 5.12±0.34×10 5 NC;
[0111] The number of nucleated cells obtained from adipose tissue preserved in normal saline was: 3.24±0.58×10 5 N.C.
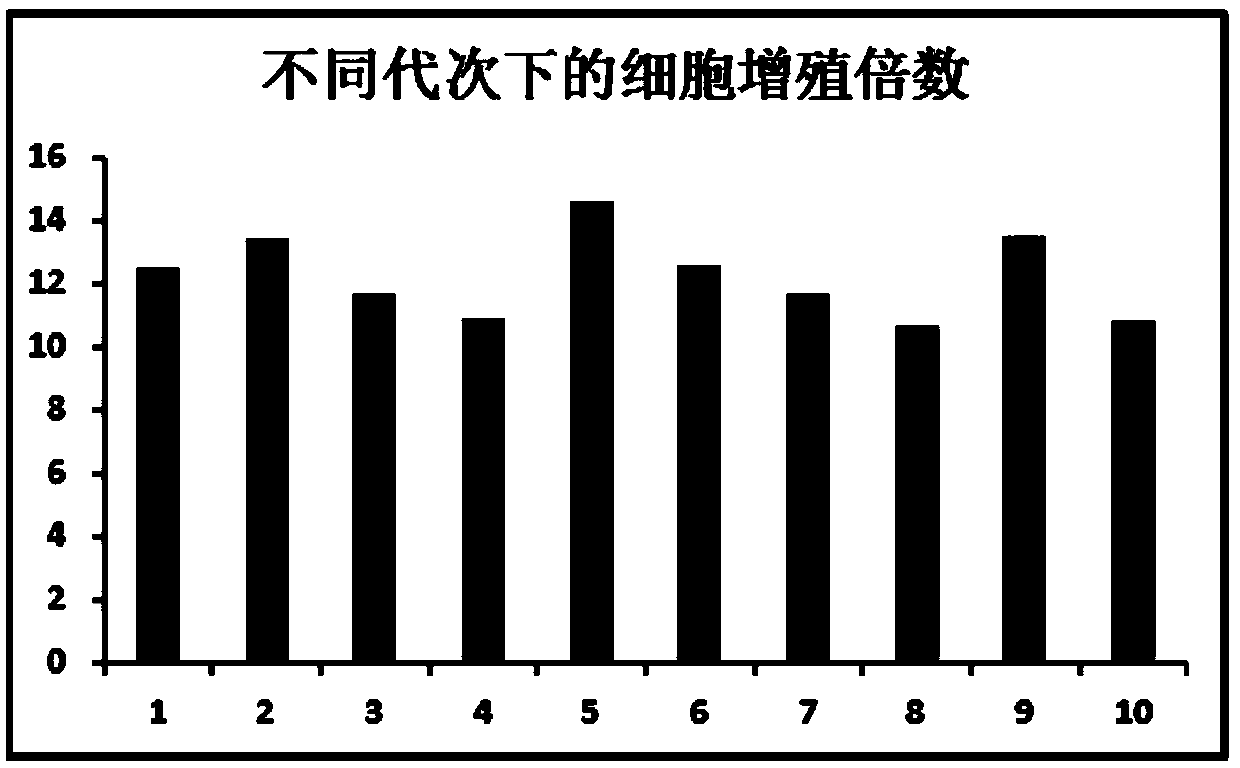
[0112] 3. Amplification times of adipose-derived stem cells
[0113] The cells after each digestion were counted, and the amplification factor of each generation was calculated. Such as figure 2 . The amplification factor of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com