Recombinant CL7-CVN protein, and preparation method and applications thereof
A CL7-CVN and protein technology, which is applied in the field of recombinant CL7-CVN protein and its preparation, can solve the problems of difficult protein expression, amino acid deletion in fusion expression, and low expression yield, and achieve good antiviral effect, high purification efficiency, and high heat The effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
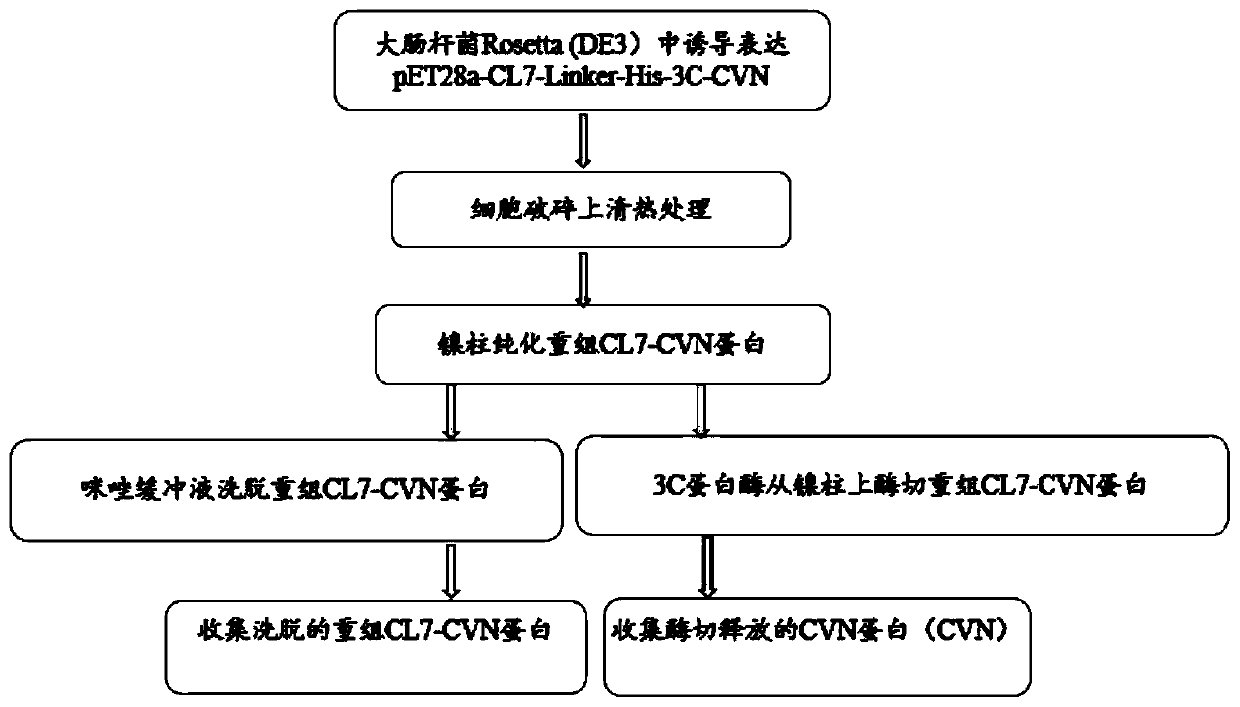
[0037] The construction of embodiment 1 recombinant CL7-CVN protein prokaryotic expression vector
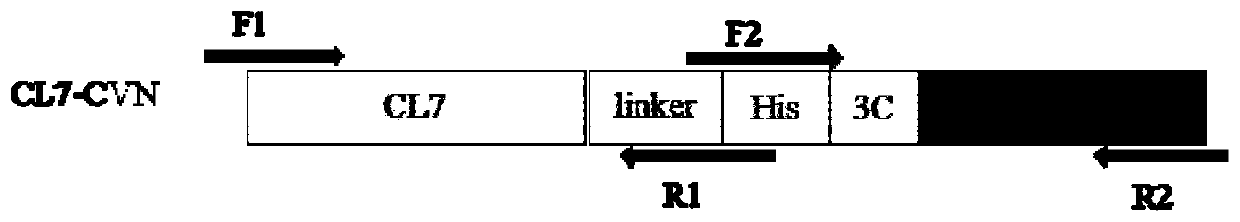
[0038] 1. The first PCR amplification: the mutant CL7 gene was obtained by site-directed mutation (the nucleotide sequence is shown in SEQ ID NO: 3, and the amino acid sequence of the mutant protein is shown in SEQ ID NO: 4);
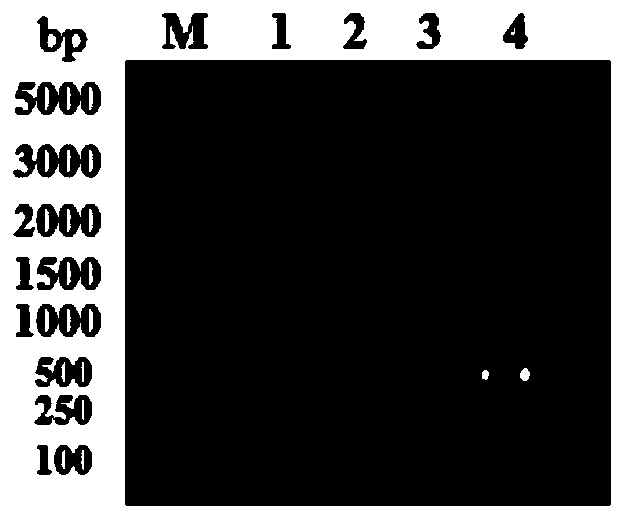
[0039] Using the pET23a-CL7 plasmid as a template, F1 shown in SEQ ID NO: 6 and R1 shown in SEQ ID NO: 7 were used as primers to simultaneously introduce linker, His and terminal homologous sequences for PCR amplification to obtain CL7- linker-His linear fragment, see image 3 Middle lane 3; according to the CVN gene sequence announced by the gene bank, carry out codon optimization design and synthesis of the CVN gene, and use the constructed pET28a-CVN vector as a template, with F2 as shown in SEQ ID NO:8 and as SEQ ID NO: R2 shown in 9 is a primer, and 3C and terminal homologous sequences are introduced at the same time, and PCR amplification is perfor...
Embodiment 2
[0049] Example 2 Induced expression of recombinant CL7-CVN protein and optimization of expression conditions
[0050] The recombinant plasmid pET28a-CL7-linker-His-3C-CVN constructed in Example 1, the empty plasmid pET28a, and the control plasmid pET28a-CVN were respectively transformed into Escherichia coli Rosetta (DE3) to obtain expression strains. The expression strain was inoculated in 6ml of liquid LB medium containing kanamycin, and cultured on a shaker at 37°C at 220rpm. When the OD600 value was 0.6, the inducer IPTG was added, and the induction culture was continued on a shaker at 37°C at 220rpm. The recombinant protein expression was detected by SDS-PAGE electrophoresis method, the results are shown in Image 6 , the induced pET28a-CL7-linker-His-3C-CVN (amino acid sequence shown in SEQ ID NO: 1) has an obvious target band at about 28kDa, which is consistent with the expected size, and the expressed protein is soluble; The CL7-labeled control pET28a-CVN showed the t...
Embodiment 3
[0055] The stability study of embodiment 3 recombinant CL7-CVN protein
[0056] Inoculate pET28a-CL7-linker-His-3C-CVN expression strains into 200ml liquid LB medium containing kanamycin, culture at 37°C until OD600 value is 0.6, add final concentration 1mM / LIPTG, and incubate at 18°C Collect the bacteria after 20 hours of induction on a shaking table; resuspend the bacteria with 20ml of lysate, break the bacteria by ultrasonic, and collect the supernatant by centrifugation; take 500ul of the supernatant in EP tubes, and place them in water bath for 15min and 30min at 90°C and 100°C respectively , 45min, 60min, 75min, 90min, 105min, 120min, the supernatant was collected by centrifugation, protein samples were prepared, and the stability of the recombinant protein was detected by SDS-PAGE electrophoresis. see results Figure 10(A, B), after 90℃, 100℃ high temperature treatment for 2 hours, most of the miscellaneous proteins were denatured, but the recombinant CL7-CVN protein w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com