Method for preparing hydroxybutanone from cellulose
A technology of hydroxybutanone and cellulose, which is applied in the field of preparation of hydroxybutanone, can solve problems such as lack of methods, and achieve the effect of simple process and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1, hydrolyzing cellulose to produce 1-hydroxyl-2-butanone and 3-hydroxyl-2-butanone
[0027] Place 1g of microcrystalline cellulose in a 100ml reaction kettle filled with sufficient water (40ml), add 0.3gWO 3 Catalyst and 0.1 g of Ru / ZrO with 3% loading 2 Catalyst, filled with H 2 , the pressure in the reactor was 40atm, heated to 240°C, and reacted for 30 minutes.
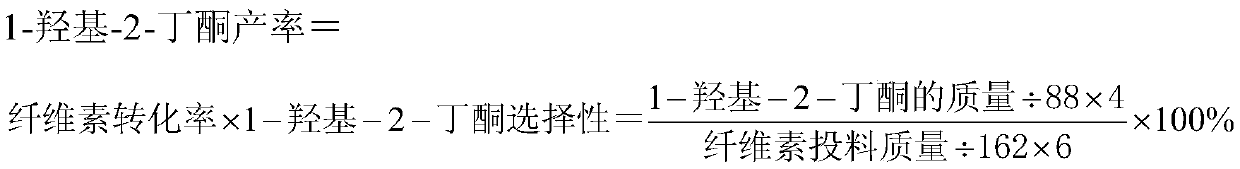
[0028] According to the following method, the conversion rate of cellulose, the yield of 1-hydroxyl-2-butanone and 3-hydroxyl-2-butanone were detected:
[0029] The mass of the unreacted cellulose was weighed on a balance, and the remaining mass was 0 g, indicating that the cellulose had been completely converted after 30 minutes of reaction. The reaction product was analyzed and quantified by high performance liquid phase (Shimadazu LC-20A HPLC; separation column: BioRad Carbonhydrate HPX-87C; analysis conditions: mobile phase: water: acetonitrile (3:2), 65°C, 0.3ml / min) , the results show t...
Embodiment 2
[0038] Embodiment 2, hydrolyzing cellulose to produce 1-hydroxyl-2-butanone and 3-hydroxyl-2-butanone
[0039] 1g of microcrystalline cellulose (microcrystalline cellulose, available from Alfa Aesar) was placed in a 100ml reaction kettle filled with sufficient water (40ml), and 0.3g WO 3 Catalyst and 0.1 g of Pt / TiO with a loading of 3% 2 Catalyst, filled with H 2 , the pressure in the reactor was 40atm, heated to 240°C, and reacted for 30 minutes.
[0040] According to the following method, the conversion rate of cellulose, the yield of 1-hydroxyl-2-butanone and 3-hydroxyl-2-butanone were detected:
[0041] The mass of unreacted cellulose was weighed on a balance, and it was 0 g. The reaction product was analyzed and quantified by high performance liquid phase (Shimadazu LC-20A HPLC; separation column: BioRad Carbonhydrate HPX-87C; analysis conditions: mobile phase: water: acetonitrile (3:2), 65°C, 0.3ml / min) , the results show that in HPLC analysis, the retention time of...
Embodiment 3
[0050] Embodiment 3, hydrolyzing cellulose to produce 1-hydroxyl-2-butanone and 3-hydroxyl-2-butanone
[0051] 1g of microcrystalline cellulose (microcrystalline cellulose, available from Alfa Aesar) was placed in a 100ml reaction kettle filled with sufficient water (40ml), and 0.3g WO 3 Catalyst and 0.1 g of Pt / Al with 3% loading 2 o 3 Catalyst, filled with H 2 , the pressure in the reactor was 40atm, heated to 240°C, and reacted for 30 minutes.
[0052] According to the following method, the conversion rate of cellulose, the yield of 1-hydroxyl-2-butanone and 3-hydroxyl-2-butanone were detected:
[0053] The mass of unreacted cellulose was weighed on a balance, and it was 0 g. The reaction product was analyzed and quantified by high performance liquid phase (Shimadazu LC-20A HPLC; separation column: BioRad Carbonhydrate HPX-87C; analysis conditions: mobile phase: water: acetonitrile (3:2), 65°C, 0.3ml / min) , the results show that in HPLC analysis, the retention time of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com