Pseudomonas aeruginosa vaccine recombinant protein reSBP-ExoU, preparation method and application
A technology of Pseudomonas aeruginosa and recombinant protein, applied in botany equipment and methods, biochemical equipment and methods, applications, etc., can solve problems such as poor adjuvant adsorption and reExoU instability, simplify the production process, facilitate Separation and purification, the effect of reducing production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0041] According to a preferred embodiment of the present invention, the recombinant protein has a 26kDa glutathione-S-transferase (GTS) tag attached to the N-terminus of the amino acid sequence shown in SEQ ID NO: 1, the The use of tags makes purification conditions mild, steps simple, and does not require the addition of denaturants, so that the purified protein can maintain its spatial conformation and immunogenicity to the greatest extent.
[0042] The above-mentioned recombinant protein can be obtained by artificial synthesis, or its coding gene can be synthesized first, and then obtained by biological expression.
[0043] In the second aspect, the present invention also provides a gene capable of encoding the above-mentioned recombinant protein.
[0044] It is well known in the art that among the 20 different amino acids that make up proteins, except that Met (ATG) or Trp (TGG) are encoded by single codons respectively, the other 18 amino acids are encoded by 2-6 codons ...
Embodiment 1
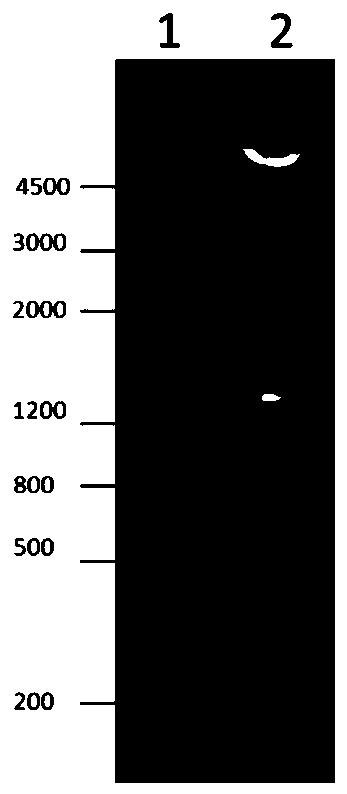
[0069] This example is used to illustrate the induction, purification and identification of the expression form of the recombinant fusion protein reSBP-ExoU in the prokaryotic expression system-Escherichia coli
[0070] (1) Synthesis and subcloning of the gene encoding reSBP-ExoU
[0071] The synthesis of the DNA sequence of reSBP-ExoU (SEQ ID NO: 2) and the connection of the DNA sequence of reSBP-ExoU and pGEX-6p-2 were synthesized by Shanghai Sangon Bioengineering Co., Ltd., thereby obtaining the recombinant plasmid pGEX-6p-reSBP- ExoU.
[0072] (2) Transformation and double enzyme digestion identification of recombinant plasmids
[0073] 1) Transformation of recombinant plasmids Escherichia coli XL1blue competent cells (Shanghai Chaoyan Biotechnology Co., Ltd.) were taken from a -80°C refrigerator, and 1 μl of recombinant plasmid pGEX-6p-reSBP-ExoU was added. Ice bath for 10 min, heat shock in a 42°C metal bath for 60 s, and quickly ice bath for 1 min. Add 600 μl of LB b...
Embodiment 2
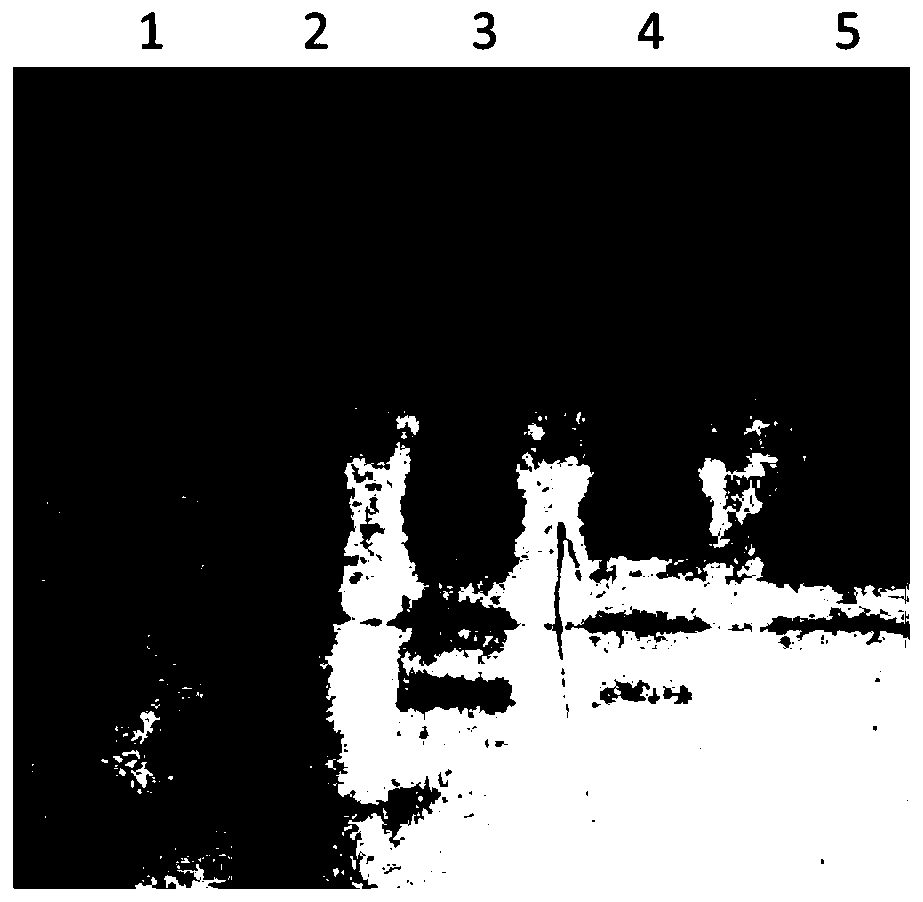
[0090] This example is used to illustrate the preparation of reSBP-ExoU antigen
[0091] (1) Amplified culture to obtain protein
[0092] Take 400 μL of the spare pGEX-6P-2-reSBP-ExoU / XL-1blue bacterial solution stored in a refrigerator at 4°C and add it to 20 mL of LB medium containing Amp resistance for primary activation. After culturing at 200 rpm at 37°C for 5-6 hours, Take 8 mL of the primary activated bacterial liquid and add it to 400 mL of LB medium containing Amp resistance for secondary activation, culture at 37°C for 3-4 hours until the OD600 is 0.6-0.8, add 80 μL of IPTG (final concentration is 200 μM) in the After induction in a shaker at 16°C for 16 hours, centrifuge at 12,000 rpm for 15 minutes to collect the bacteria, add 20 mL of lysis buffer (same as Example 1) to resuspend the bacteria, and ultrasonically lyse the bacteria for 15 minutes, collect the supernatant and 800 μL for fusion The Glutathione Sepharose4B (GE Company) filler (beads) binding treatment...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com