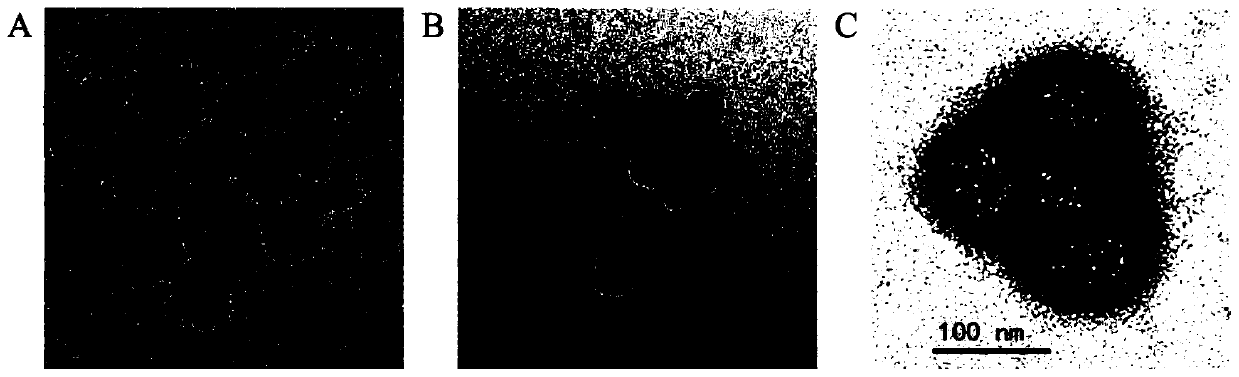
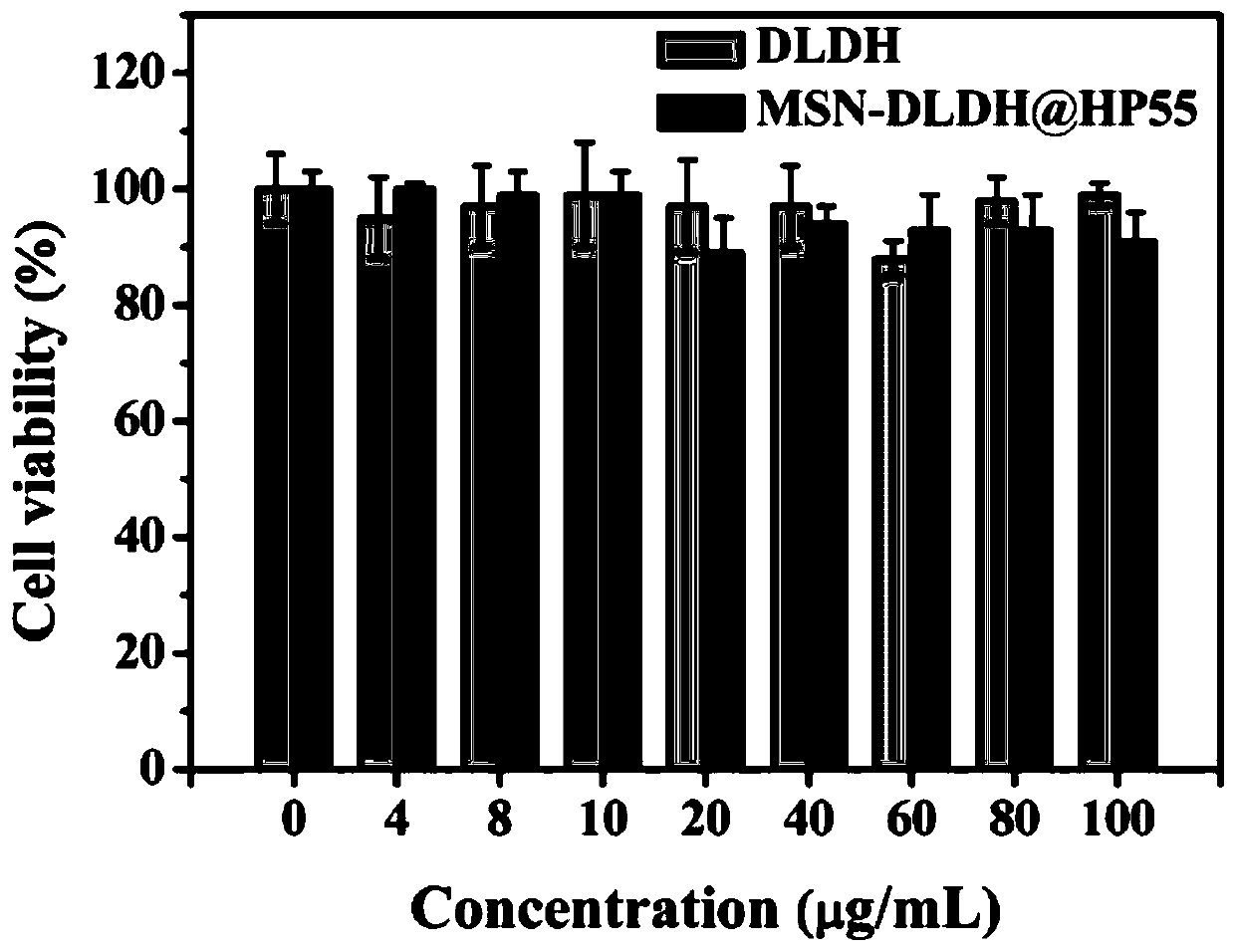
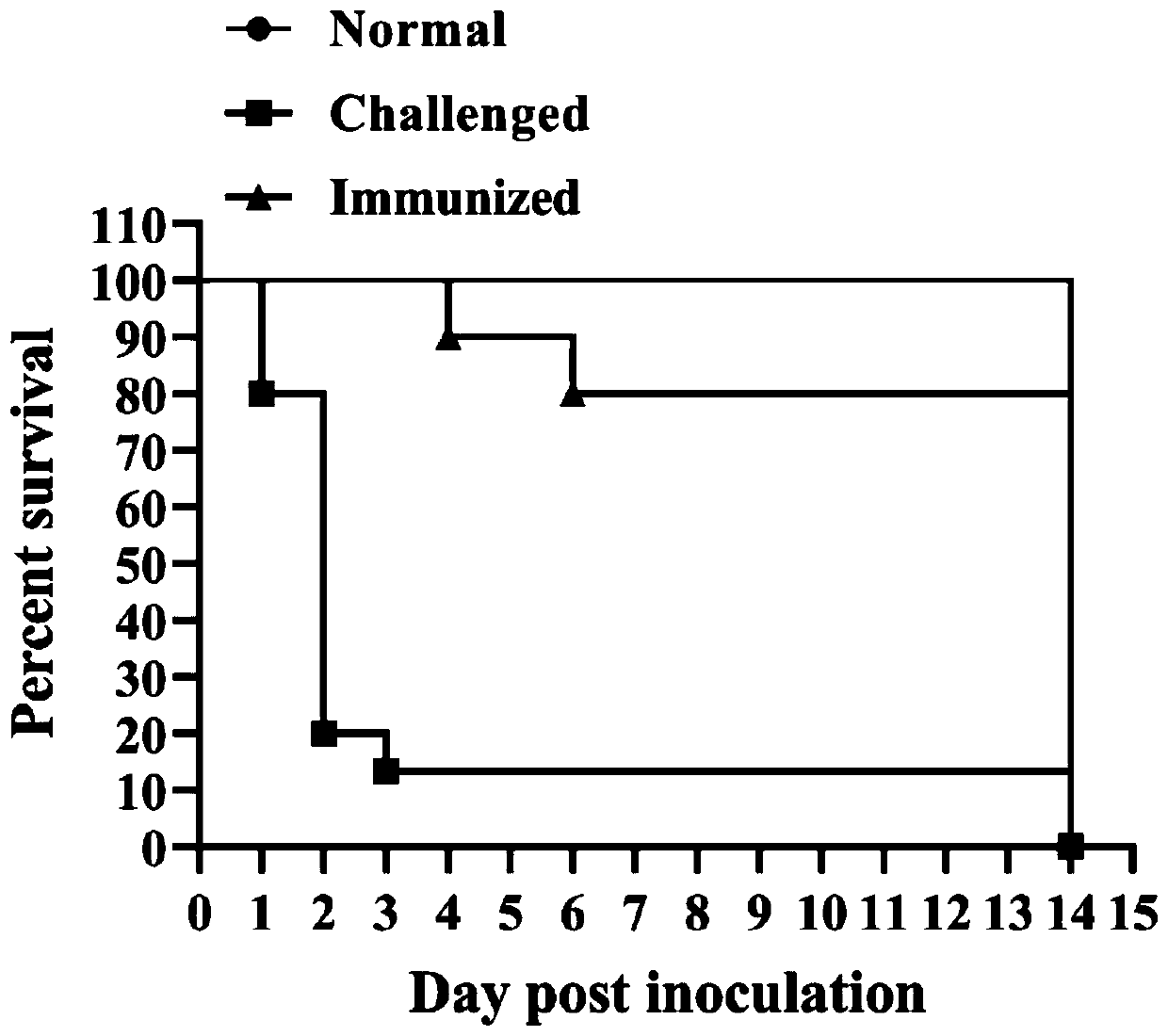
Oral nano-vaccine of large yellow croaker and immune type large yellow croaker feed prepared by the same
A nano-vaccine, large yellow croaker technology, applied in vaccines, veterinary vaccines, pharmaceutical formulations, etc., can solve the problems of inability to achieve immune effects, affect immune protection effects, etc., achieve cost-effective, reduce toxicity and stimulation, and biocompatibility Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The preparation method of described large yellow croaker oral nano-vaccine, it comprises the following processing steps:
[0027] (1) preparing MSN nanoparticles;
[0028] The specific method of step (1) is: add hexadecyltrimethyl-p-toluenesulfonate amine, triethanolamine and water into a round bottom flask, stir and dissolve at 80-85°C for 1-2h; then quickly add tetrasilicic acid Ethyl ester, continue stirring for 2-3h; centrifuge at 12745g for 20-25min to recover the precipitate; use ddH for precipitation 2 Wash twice with O, followed by 6 g / L NH at 60-65 °C 4 NO 3 The methanol solution extracted the product 3 times; after the extraction, the precipitate was recovered by centrifugation at 12745g for 20-25min, and the 2 O washed and precipitated 2 times, and the precipitate was dried at normal temperature to obtain the MSN nanoparticles.
[0029] (2) Preparation of MSN-DLDH nanoparticles: resuspend the MSN nanoparticles obtained in step (1) in ddH 2 In O, form the...
Embodiment 1
[0041] Embodiment 1: Preparation of oral nano-vaccine of large yellow croaker:
[0042] (1) Preparation of MSN nanoparticles: Add 4.8g of cetyltrimethyl-p-toluenesulfonate, 0.7mL of triethanolamine and 250mL of water into a round bottom flask, stir and dissolve at 80°C for 1h; then quickly add 10mL of silicic acid Tetraethyl ester, continue to stir and react for 2h; centrifuge at 12745g for 20min to recover the precipitate; use ddH for precipitation 2 Washed twice with O, followed by 6 g / L NH at 60 °C 4 NO 3 The methanol solution extracted the product 3 times; after the extraction, the precipitate was recovered by centrifugation at 12745g for 20min, and the 2 O washed and precipitated 2 times, and the precipitate was dried at normal temperature to obtain the MSN nanoparticles.
[0043] (2) Preparation of MSN-DLDH nanoparticles: resuspend the MSN nanoparticles obtained in step (1) in 20mL ddH 2 In O, the MSN nanoparticle suspension liquid that obtains concentration is 23.2m...
Embodiment 2
[0048] Embodiment two: the preparation of immune type large yellow croaker feed:
[0049] Resuspend the MSN-DLDH@HP55 enteric-coated nanoparticles obtained in step (3) of Example 1 in 200mL ddHO 2 In O, a suspension of MSN-DLDH@HP55 enteric nanoparticles with a concentration of 1.77mg / mL was formed;
[0050] MSN-DLDH@HP55 vaccine mix: Next, mix 2kg of large yellow croaker No. 2 feed with 3g of adhesive, and then evenly spray MSN-DLDH@HP55 enteric-coated nanoparticle suspension on the surface of large yellow croaker No. 2 feed Stir evenly again, spray 1mL of MSN-DLDH@HP55 enteric-coated nanoparticle suspension per 20g of large yellow croaker No. 2 feed, and then vacuum freeze-dry at -30°C to obtain feed with MSN-DLDH@HP55 attached. The feed with MSN-DLDH@HP55 was soaked in the chitosan solution for 3 seconds to form a thin coating (the chitosan solution should not cover the feed with MSN-DLDH@HP55 during soaking), and quickly transferred to ddH 2 Soak in O for 5s and take out...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com