Mutant of nitrile hydratase derived from caldalkalibacillus thermarum
A technology of nitrile hydratase and nitrile hydratase is applied in the field of enzyme engineering and can solve the problems of low concentration of final product, long growth cycle of Rhodococcus, low production efficiency and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
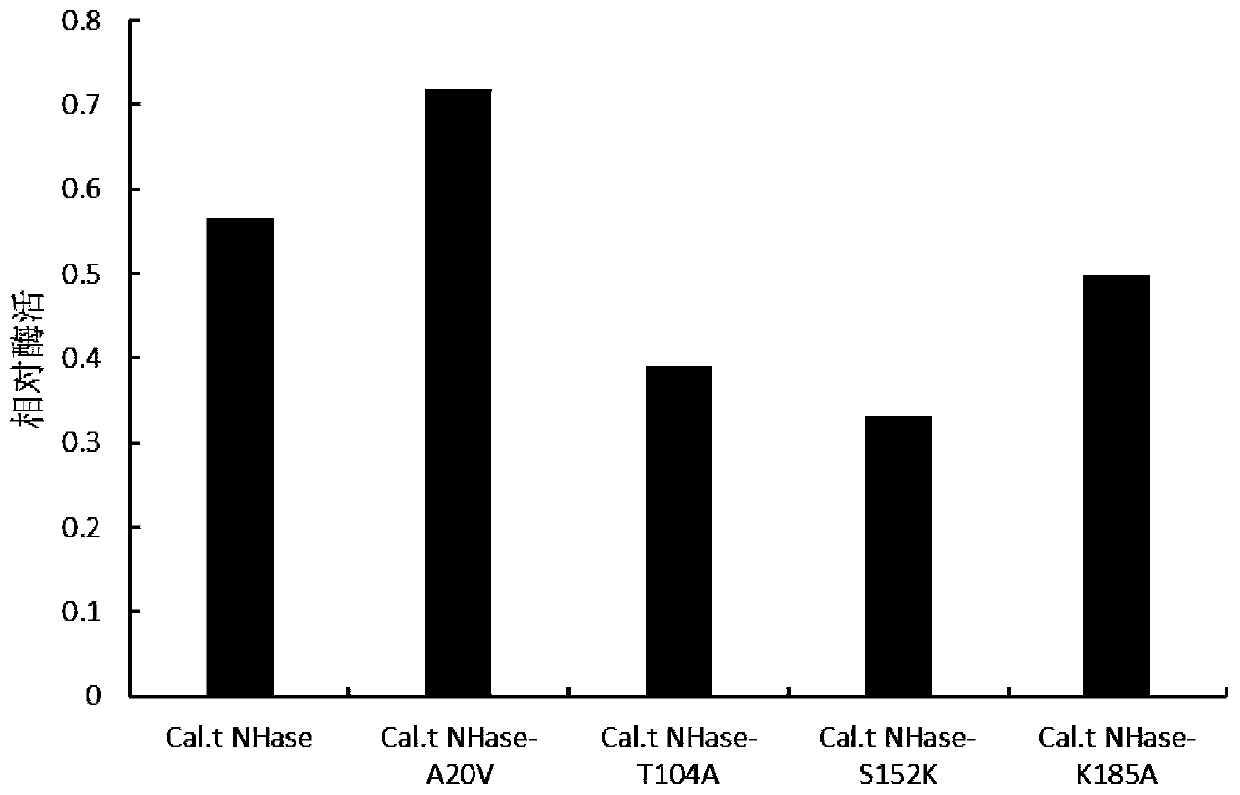
[0035] Kinetic simulation of nitrile hydratase (PtNHase) derived from Pseudonocardia thermophila and Cal.t NHase derived from Caldalkalibacillus thermarum found that the RMSF value of some amino acids was higher than that of It is speculated that these amino acids may affect its thermal stability. Therefore, the following mutants were constructed: Cal.t NHase-A20V, Cal.t NHase-H150S (the histidine at the 150th position of the β subunit shown in SEQ ID NO.2 is mutated into serine in the amino acid sequence) , Cal.t NHase-T104A (the threonine at the 104th position of the β subunit shown in SEQ ID NO.2 is mutated to alanine), Cal.t NHase-S152K (the amino acid sequence is as shown in SEQ ID NO.2 The serine at the 152nd position of the β subunit shown in .2 is mutated into lysine), Cal.t NHase-K185A (the lysine at the 185th position of the β subunit shown in the amino acid sequence as SEQ ID NO.2 is mutated to alanine).
[0036] (1) Construction of mutants:
[0037] Synthesize t...
Embodiment 2
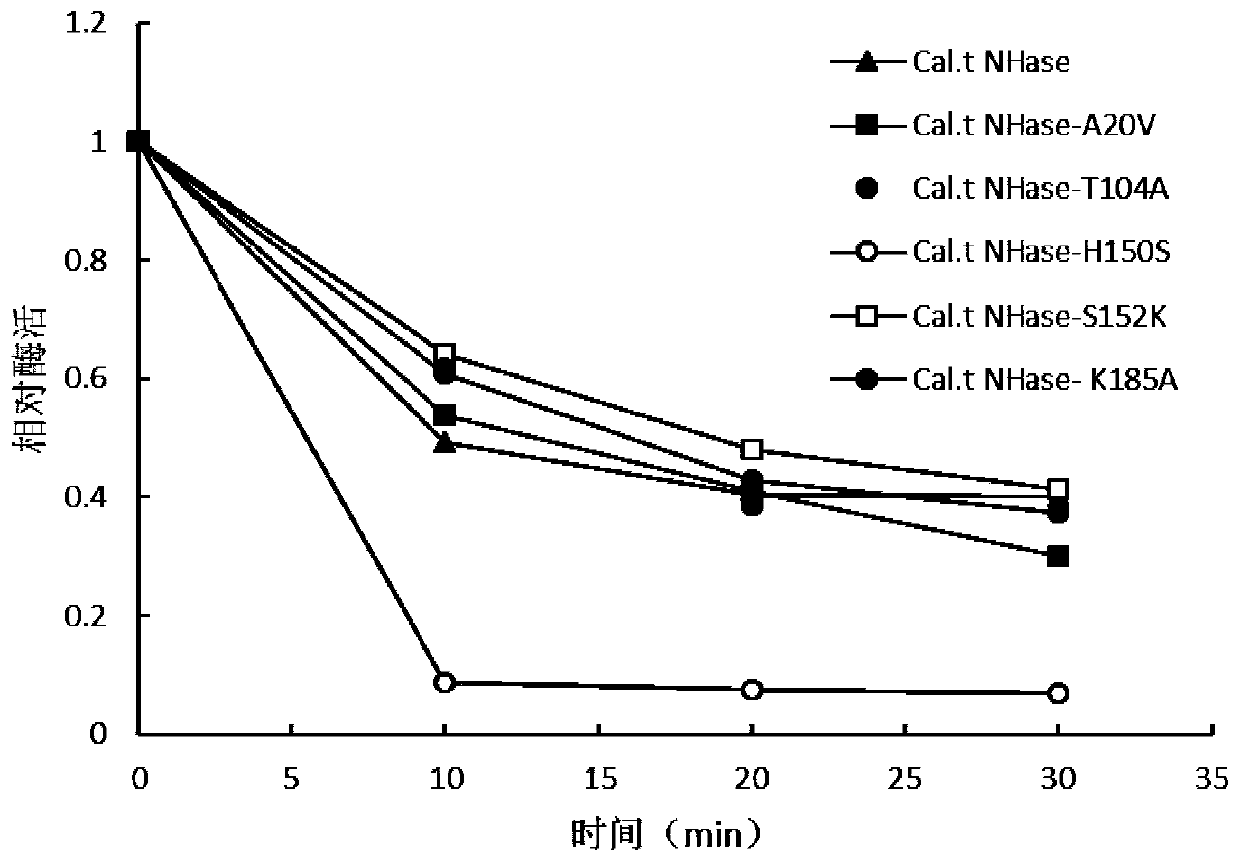
[0049] Add 10 μL of 0.5 mg / mL mutant enzyme purified in Example 1 to the 500 μL buffer reaction system, and treat it in a metal bath at 70°C for 0 min, 10 min, 20 min, and 30 min, respectively, and measure the residual enzyme activity. is 100%.
[0050] like figure 1 As shown, it was found that the mutant enzyme Cal.t NHase-H150S was treated at 70°C for 10 minutes, and the enzyme activity of the mutant enzyme Cal. Properties of the tNHase-H150S mutant enzyme.
Embodiment 3
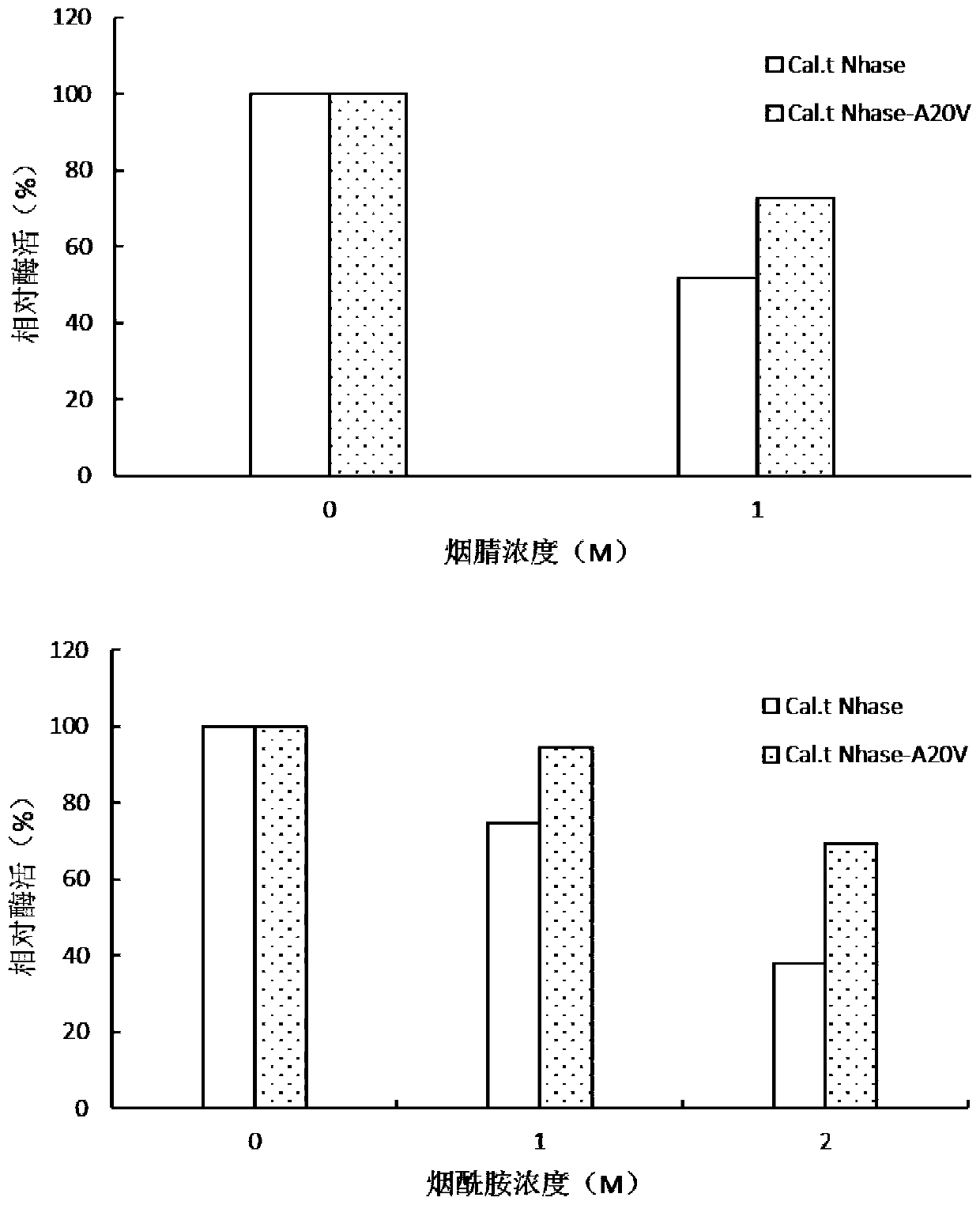
[0052] Prepare different concentrations of product nicotinamide solution: 0M, 2M, OD 600 =8 The wild enzyme and the mutant bacterial solution were respectively treated in solutions with different substrate concentrations at 30°C for 30 minutes, then the cells were resuspended and washed twice with KPB, and 10 μL was taken to measure the residual enzyme activity. The enzyme activity treated with 0M was defined as 100%.
[0053] like figure 2 As shown, the enzyme activity without product treatment is defined as 100%. It was found that after the mutant was treated with 2M product nicotinamide for 20 minutes, the remaining enzyme activity of the mutant enzyme Cal.t Nhase-A20V was increased from 40% of the wild enzyme to 69%. %, and the rest of the mutant enzymes Cal.t NHase-H150S, Cal.t NHase-T104A, Cal.t NHase-S152K, Cal.t NHase-K185A, compared with wild enzymes, showed different degrees of decline. The tolerance of the mutant enzyme Cal.t Nhase-A20V product was significantly ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com