Application of oxidoreductase and mutant thereof in biosynthesis of noottanone
A biosynthesis and reductase technology, applied in the field of bioengineering, can solve the problems of unsatisfactory industrial production, low substrate concentration, poor salt tolerance, etc., and achieve excellent effect, good substrate tolerance, and good substrate tolerance. degree of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
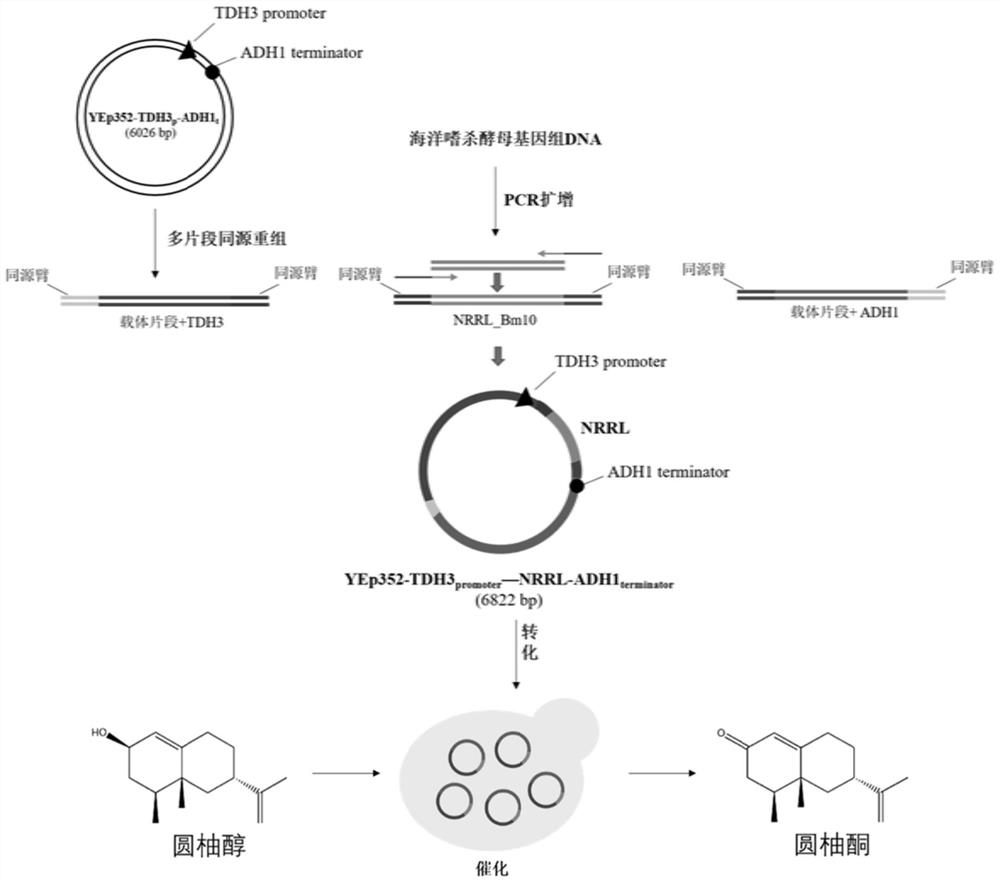
[0041] Example 1: Cloning of oxidoreductase NRRL and construction of expression vector
[0042] The marine killing yeast Wickerhamomyces anomalus M15 was inoculated in 5mL YPD (glucose 20g / L, yeast extract 10g / L, peptone 20g / L) liquid medium and cultured at 30°C until logarithmic growth phase. Genomic total DNA was extracted from the marine killing yeast using the Yeast DNAKit kit (purchased from Omega).
[0043] Aiming at the hypothetical protein [named WICANDRAFT_92107 (NCBI accession number is XP_019039214.1) derived from marine killer yeast (Wickerhamomyces anomalus NRRL Y-366-8), it contains a total of 264 amino acids; its coding gene (NCBI accession number is XM_019186339. 1) The nucleotide sequence contains a total of 795 nucleotides] A pair of specific primers are designed for the coding gene sequence, and each primer introduces a base sequence homologous to the vector at the 5' end for homologous recombination Cloning method To construct the YEp352 expression vector,...
Embodiment 2
[0062] Example 2: Construction of recombinant expression cells
[0063]Using the yeast transformation kit S.c.Easy Comp Transformation Kit (purchased from Invitrogen, USA), the recombinant expression plasmid YEp352-TDH3 constructed in Example 1 was p -NRRL-ADH1 t Transformed into S.cerevisiae CEN.PK2-1 Ca competent cells.
[0064] 1) Add 500ng of recombinant plasmid and 250μL of Solution III (Transformation solution) to every 25μL of competent cells;
[0065] 2) Oscillate evenly with a vortex shaker, and place in a constant temperature incubator at 30°C for 15 minutes;
[0066] 3) Shake and mix again after taking it out, place it at 30°C for 15 minutes, and repeat the operation twice;
[0067] 4) Then take 200 μL of recombinant yeast cells and spread them evenly on the auxotrophic plate SD / ΔUra (6.7g / L YNB, 2g / L amino acid mixture, 20g / L glucose, 20mg / L leucine, 20mg / L tryptophan) Spread the plate and incubate at 30°C for 2 to 4 days.
[0068] Until a single clone grows o...
Embodiment 3
[0069] Example 3: Catalytic preparation of naringone by whole cells in vitro
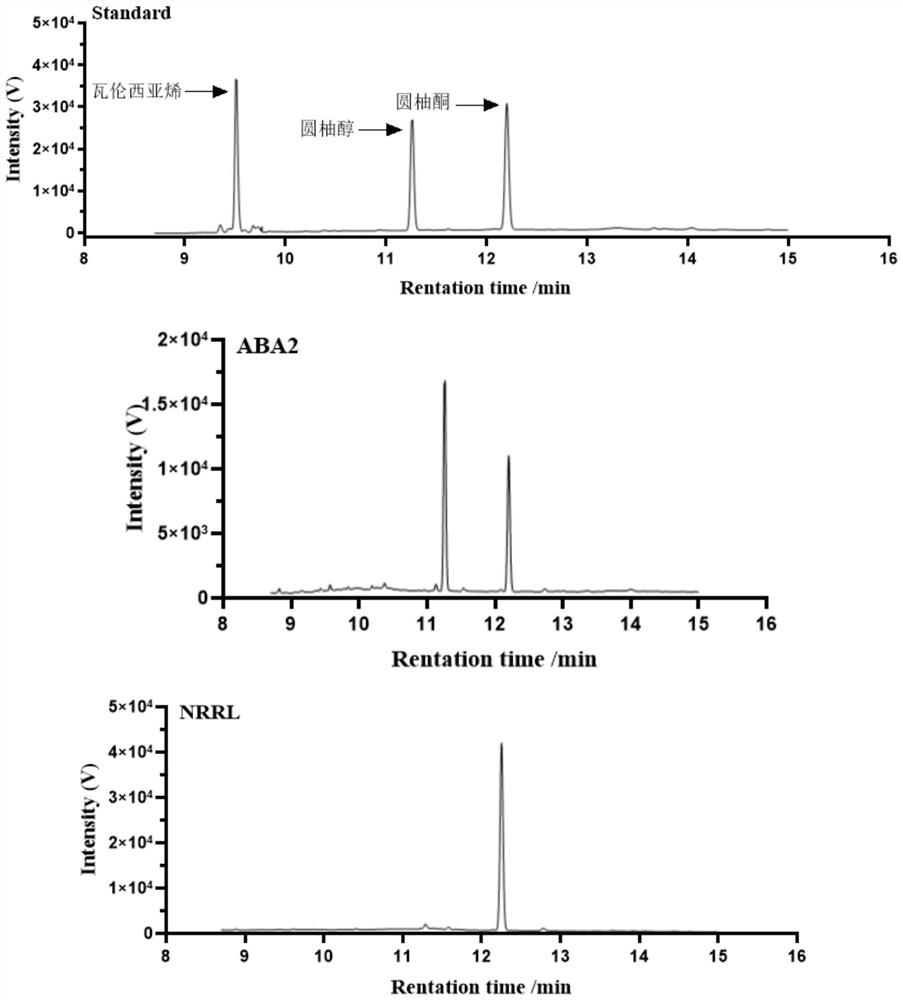
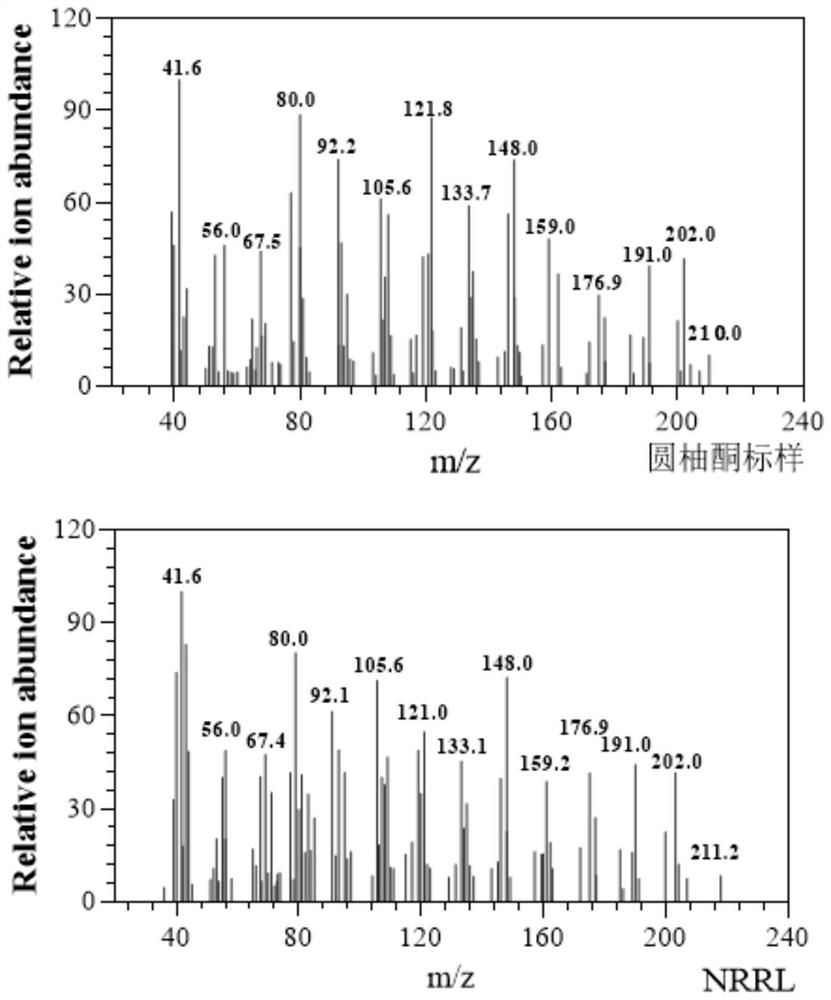
[0070] Pick the recombinant expression cell S.c.CEN.PK2-1 Ca (YEp352-TDH3 p -NRRL-ADH1 t ) into a test tube containing 5 mL of SD / ΔUra liquid medium, and cultured at 30° C. and 220 rpm for 24 hours. Then press initial OD 600 The inoculum size =0.05 was transferred to a 250 mL shake flask filled with 50 mL SD / ΔUra liquid medium, and cultured at 30° C. and 220 rpm for 24 hours. Subsequent collection of total OD 600 Centrifuge at 3000rpm, 4°C for 5min, and discard the supernatant. Then the recombinant expression cells were resuspended to 1mL with potassium phosphate buffer (50mM, pH 7.4) to obtain 50OD 600 / mL of reaction solution. Add 20 μL of 100 mM grapefruit alcohol solution (containing 1% (v / v) Triton-100, dissolved in dimethyl sulfoxide) to make the final substrate concentration 2 mM, and catalyze at 25° C. and 220 rpm for 24 hours.
[0071] Product detection method is as follows (the prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com