AMA synthetase and application thereof in synthesis of AMA or derivatives thereof
A technology for synthesizing enzymes and derivatives, applied in the field of genetic engineering, which can solve the problems of harsh reaction conditions of chemical synthesis, inability to meet the needs of industrialization, and difficulty in obtaining natural products, etc., to achieve high atom economy and environmental friendliness, and good results Drug tolerance, easy purification effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0105] Cloning of embodiment 1 AMA synthetase gene
[0106] RNA extraction: The lyophilized powder of Aspergillus oryzae strain A.oryzae 3.9544 was reconstituted with sterile 0.8M NaCl, cultured in PDA medium for three days, and spores were collected with 0.8M NaCl. The spore liquid was inoculated in 100ml CD liquid medium, and the spore concentration was 2×10 8 / ml, and then cultivated at 30°C with shaking at 220rpm. Two weeks later, the Aspergillus oryzae RNA was extracted with the Plant Total RNA Extraction Kit from Tiangen Biochemical Co., Ltd.
[0107] cDNA preparation: the extracted RNA was treated with DNAase at 37°C for half an hour, then purified with an RNA purification kit, and the recovered concentration was determined by nanodrop. cDNA was obtained by reversing with Thermo Scientific RevertAid First Strand cDNASynthesisKit.
[0108] Amplification of the AMA synthetase gene sequence:
[0109] Primer sequence: Pet29a-AMA Synthetase-F: GGGAATTCCATATGGCCAATCTCAATG...
Embodiment 2
[0118] The expression purification method of embodiment 2 AMA synthetase
[0119] Transform the Escherichia coli BL21(DE3) strain with pET29a-AMA synthetase, inoculate the resulting transformants into liquid LB medium for culture, and transfer 20 mL to 1 L of LB medium containing 50 μg / ml kanamycin , 37°C, 220rpm shaking culture until the OD of the bacterial solution is 0.8-1.0, and after cooling at 16°C for 1h, add IPTG with a final concentration of 0.5mM for 18-20h induction. After the cultivation, the bacterial cells were recovered by centrifugation (5000×g, 40 minutes), and the bacterial cells were resuspended with lysis buffer (50 mM Tris, 500 mM NaCl, pH 7.4). Ultrasonic treatment was performed to crush the bacteria (500W, 30% power, ultrasonic for 5s, stop for 5s, and crush for 1h in total). Then centrifuge (15000rpm, 1h) to remove the cell residue, collect the supernatant and filter it with a 0.22μm filter membrane to obtain a crude enzyme solution. The nickel column...
Embodiment 3
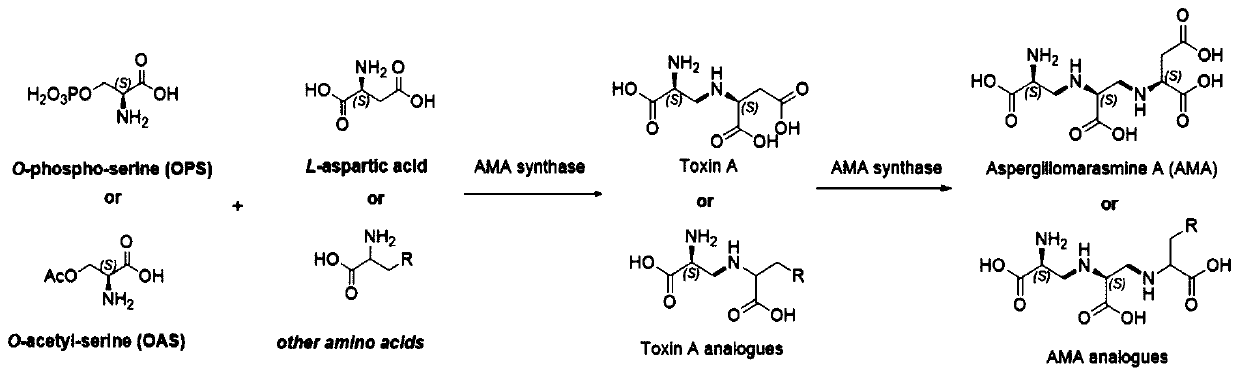
[0120] Example 3 AMA synthetase catalyzes the reaction of oxyphosphoserine (OPS) / oxyacetylserine (OAS) and L-aspartic acid
[0121] The AMA synthetase obtained in Example 2 was used. AMA synthetase catalyzes the reaction process of OPS / OAS and L-aspartic acid, see image 3 .
[0122] AMA synthase activity was assayed as follows: 20 μM AMA synthase, 10 mM oxyphosphoserine / oxyacetylserine. 10 mM potassium L-aspartate, 1 mM PLP, 2 mM TCEP. Reaction at room temperature for 16h. Detection method: Take 50μl of the above reaction solution, add 50μl 100mM Fmoc-NHS, 50μl 0.2M boric acid (pH10.0), 50μl acetonitrile, react for 6h, then quench the reaction with 50μl methanol. After filtering through a 0.22 μm filter membrane, carry out UPLC-MS detection with the following liquid phase method: 0-0.3min: 5% acetonitrile, 95% water (containing 0.1% formic acid); 0.3-4min: the concentration gradient of acetonitrile increases to 50%, The concentration gradient of the aqueous phase is redu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com