Gallocatechin and gallocatechin in preparing intestinal pathogenic bacterium medicine and feed additive
A gallocatechin and feed additive technology, applied in the field of veterinary medicine, can solve problems such as the mismatch between the serotype of pathogenic bacteria and the serotype of the vaccine, and the poor prevention and control effect of E. effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Determination of minimum inhibitory concentration and minimum bactericidal concentration of gallocatechin on Escherichia coli K88, K99, 987P:
[0025] It can be seen from Table 1 that when the concentration of gallocatechin is 256 μg / ml, it has an inhibitory effect on the growth of intestinal pathogenic bacteria, and when the concentration of gallocatechin is 512 μg / ml, it has a bactericidal effect on intestinal pathogenic bacteria.
[0026] Table 1 gallocatechin minimum inhibitory concentration and minimum bactericidal concentration to Escherichia coli K88, K99, 987P:
[0027]
[0028] Table 1: Minimum inhibitory concentration and minimum bactericidal concentration of gallocatechin on Escherichia coli K88, K99, 987P
Embodiment 2
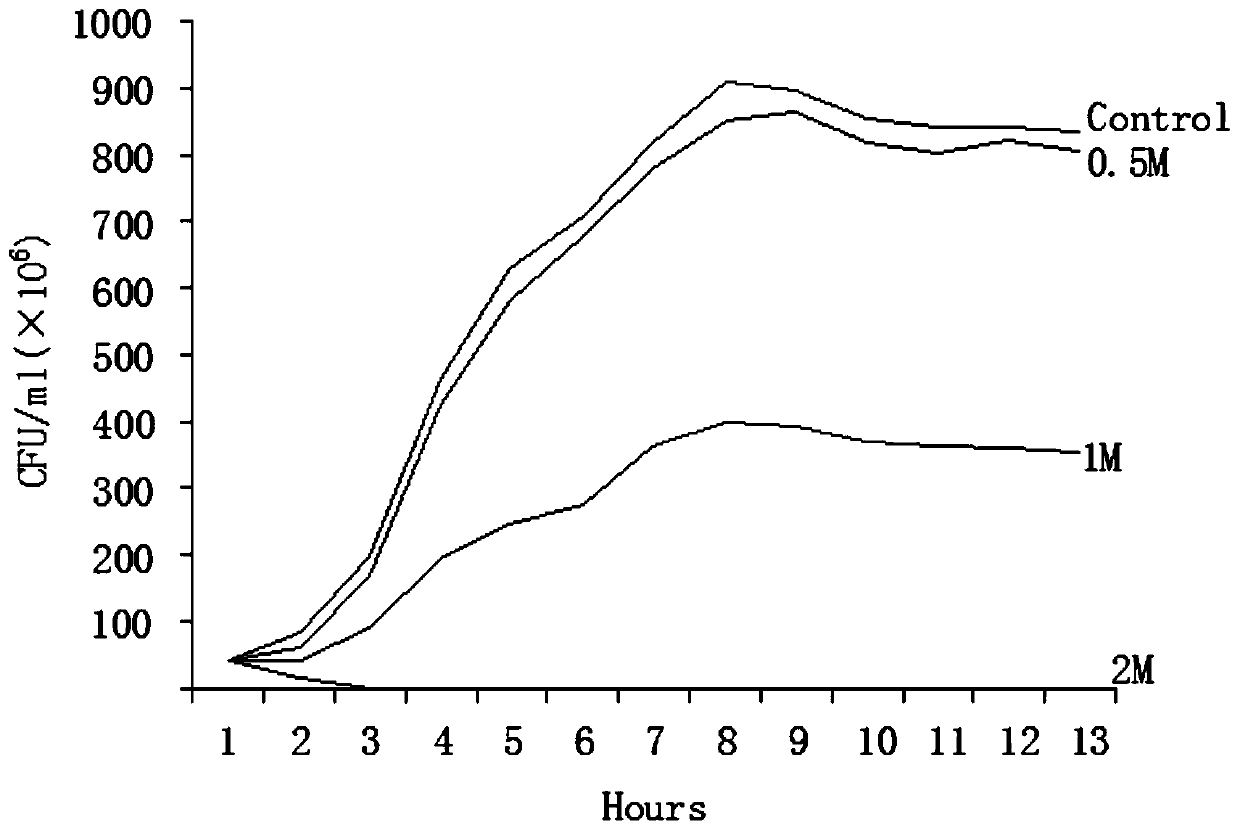
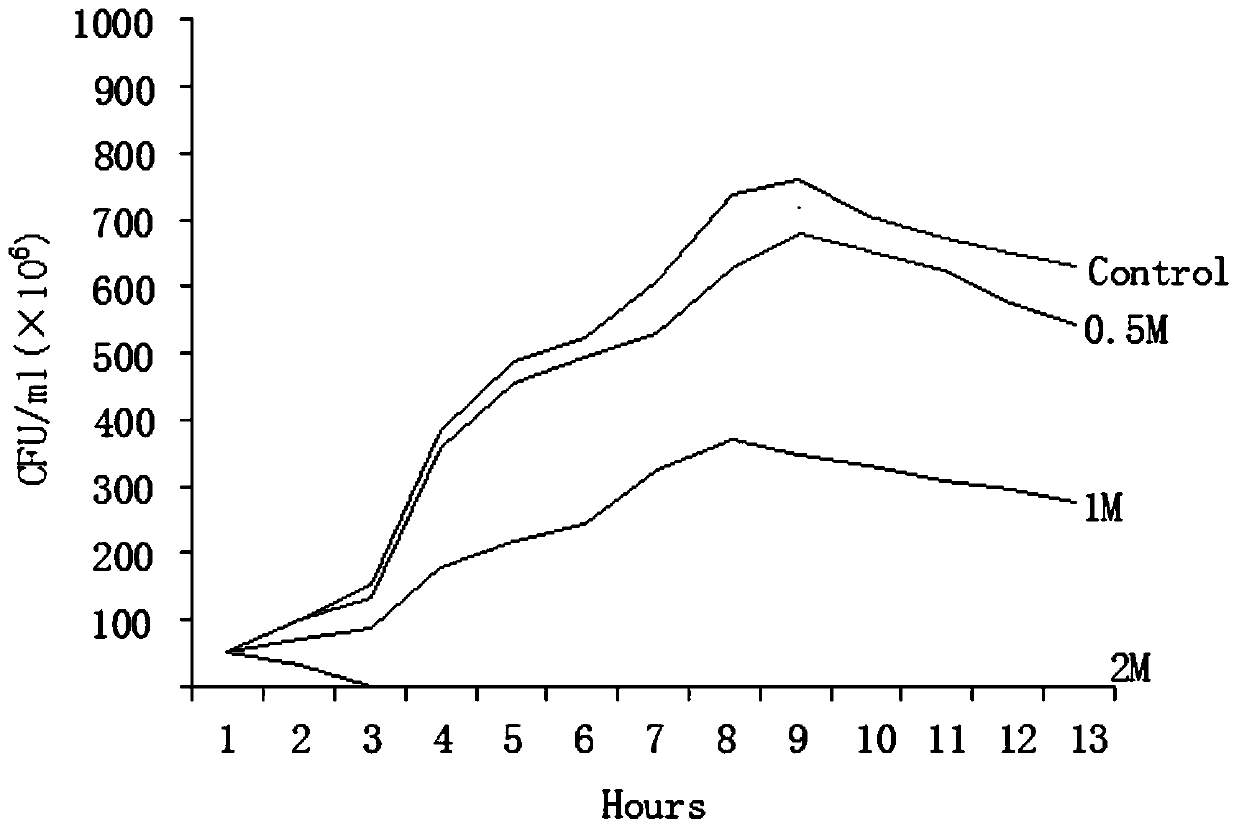
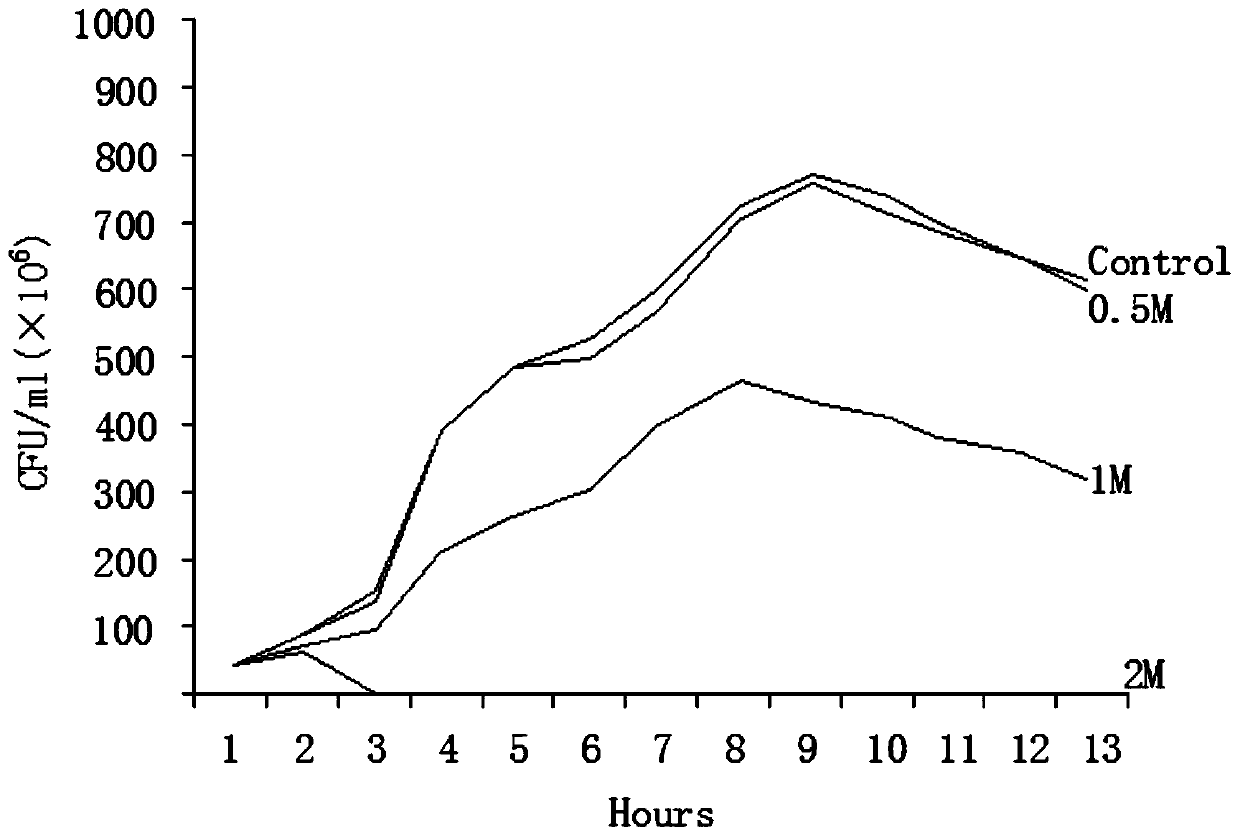
[0030] Inhibitory effect of gallocatechin on the growth curve of Escherichia coli K88, K99, 987P:
[0031] Purified colonies of Escherichia coli K88, K99, and 987P were picked out with a sterile inoculation loop, fully dispersed in MacConkey liquid medium, and a McFarland turbidimetric standard bacterial suspension with a concentration of 0.5 was prepared by the McFarland turbidimetric method.
[0032] Gallocatechin solutions with different concentrations were added to sterile bacterial bottles after doubling dilution with MacConkey liquid medium, and the growth control only had MacConkey liquid medium without gallocatechin solution.
[0033] Inoculate an equal amount of 0.5 McFarland turbidimetric standard bacteria suspension into the above-mentioned bacterial bottle, place it on a 37-degree shaker for shaking culture, take the bacterial solution and apply it on a MacConkey plate every two hours to count the viable bacteria. The results are expressed in CFU / expressed in ml. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Minimum inhibitory concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com