A kind of catalyst and preparation method for synthesizing propylene glycol ether
A propylene glycol ether and catalyst technology, which is applied in ether preparation, chemical instruments and methods, organic compound/hydride/coordination complex catalysts, etc., can solve the problems of difficult catalyst recovery, strong corrosion, and large amount of three wastes generated
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
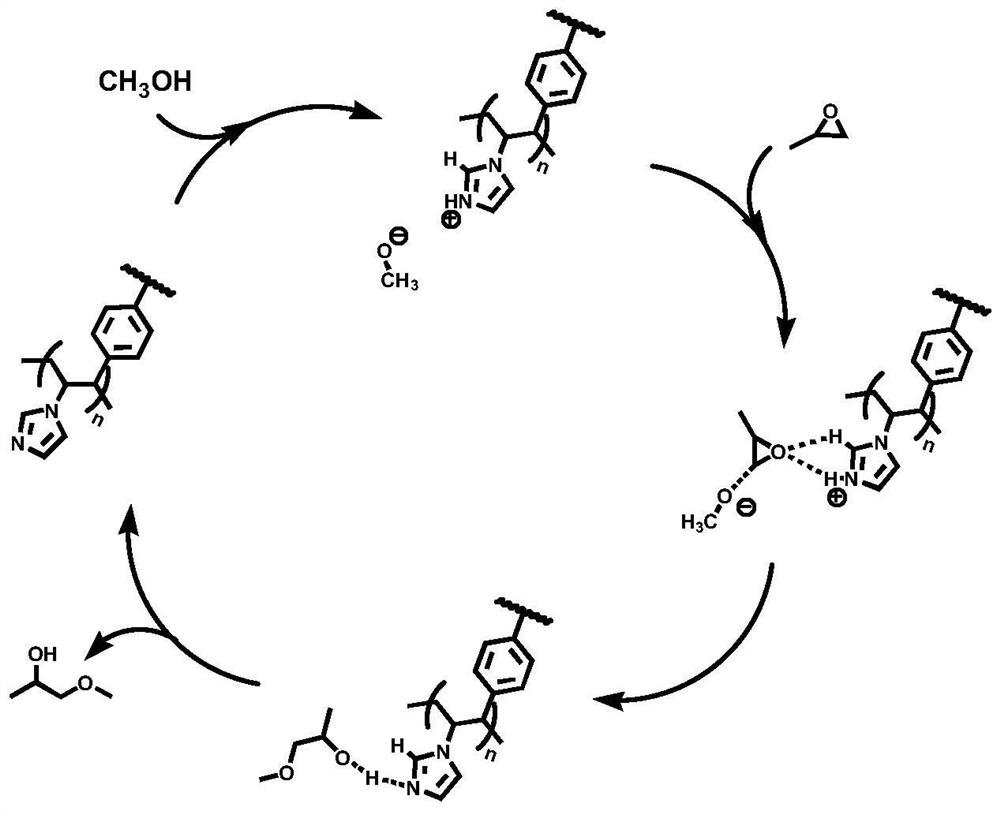
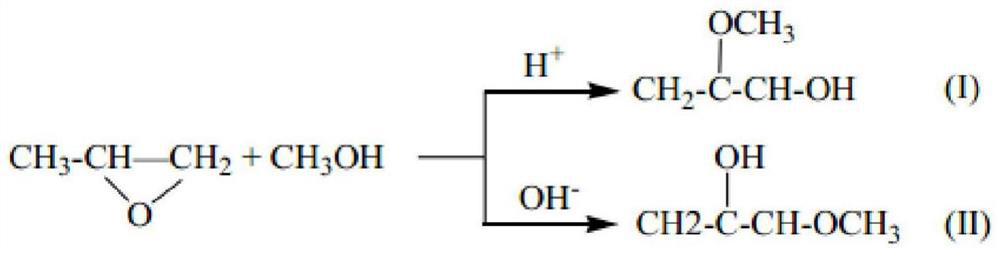
[0016] The embodiment of the present invention also provides the preparation method of the above-mentioned catalyst for synthesizing propylene glycol ether, the method comprises the following steps:
[0017] Add azobisisobutyronitrile to the ethyl acetate solution of 1-vinylimidazole with a concentration of 0.5~1.2mol / L, N 2 Or heated to 65-90°C under inert gas, kept for 0.5-2h, then added divinylbenzene ethyl acetate solution, kept at 65-90°C for 12-24h, cooled, and the product was dried to obtain the synthetic propylene glycol ether The catalyst, wherein the azobisisobutyronitrile is added in an amount of 0.01-5 wt% of 1-vinylimidazole, and the molar ratio of 1-vinylimidazole to divinylbenzene is 1:0.01-20.
[0018] Preferably, the added amount of azobisisobutyronitrile is 0.2-0.6 wt% of 1-vinylimidazole, and the molar ratio of 1-vinylimidazole / divinylbenzene is 1:0.2-3. More preferably, the added amount of azobisisobutyronitrile is 0.3-0.5 wt% of 1-vinylimidazole, and the ...
Embodiment 1
[0022] Synthesis of polyvinylimidazole-divinylbenzene (PVIM-DVB):
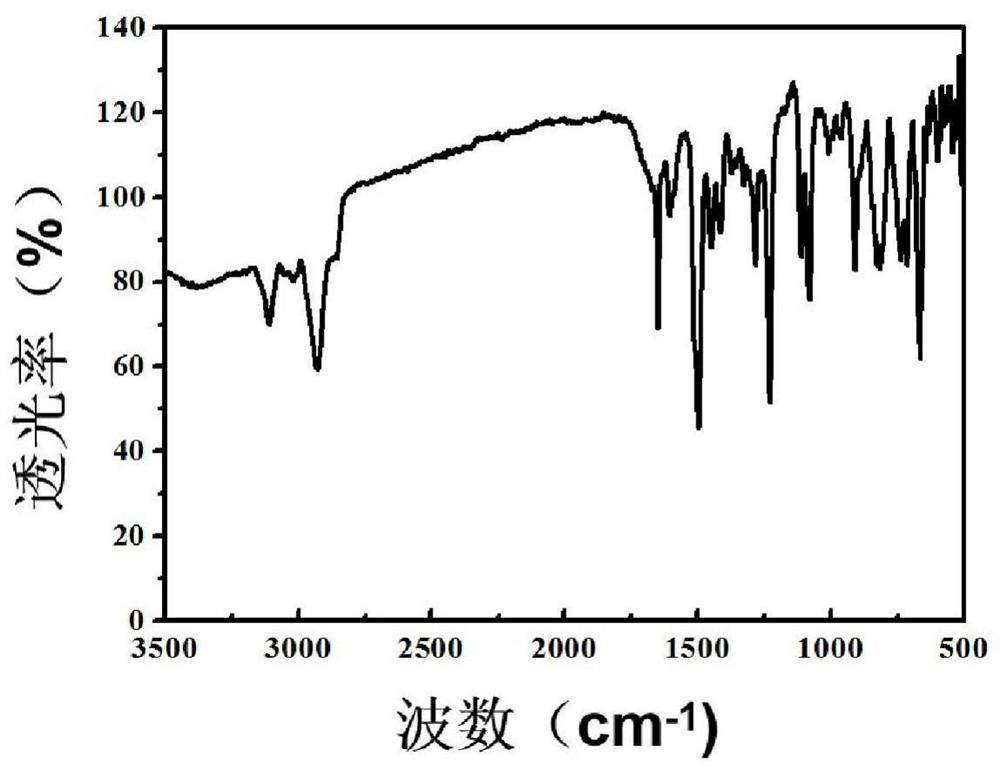
[0023] 2.3g vinylimidazole, 0.02g azobisisobutyronitrile dissolved in 30mL ethyl acetate, N 2 Purge and replace air for 30min, and heat to 90°C for 1h; then add 2.3g of divinylbenzene in ethyl acetate solution dropwise, react for 24h, cool to room temperature, move to a vacuum drying oven, and dry at 60°C for 24h , to obtain pale yellow solid PVIM-DVB. Its infrared characterization is as follows figure 2 , showing the characteristic absorption peaks of VIM and DVB units, 1650cm -1 The peak at is the stretching vibration peak of the imidazole unit C=N bond, 1500cm -1 The peak at is the stretching vibration peak of the C=C bond between the imidazole ring and the benzene ring, 900cm -1 、650cm -1 The peak at is the out-of-plane stretching vibration peak of the C-H bond on the benzene ring. The appearance of these characteristic peaks fully shows that the monomer VIM and DVB have been cross-linked and copoly...
Embodiment 2
[0025] Synthesis of polyvinylimidazole-divinylbenzene (PVIM-DVB):
[0026] 2.3g vinylimidazole, 0.02g azobisisobutyronitrile dissolved in 30mL ethyl acetate, N 2 Purge and replace the air for 30min, and heat to 90°C for 1h; then add dropwise an ethyl acetate solution containing 4.6g of divinylbenzene, react for 24h, cool to room temperature, move to a vacuum drying oven, and dry at 60°C for 24h , to obtain light yellow solid PVIM-DVB.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com