Hyaluronic acid buccal tablet and preparation method thereof
A technology of hyaluronic acid and buccal tablets, which can be applied to medical preparations containing active ingredients, pharmaceutical formulas, and medical preparations without active ingredients, which can solve the problems of storage stability, safety, lack of portability, and efficacy Problems such as limited parts and high irritation, to achieve outstanding effects, reduce irritation, and easy absorption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0086] This paper also relates to a preparation method of the above-mentioned hyaluronic acid buccal tablet, which is characterized in that: comprising the following steps: adding high molecular weight hyaluronic acid or its salt, low molecular weight hyaluronic acid or its salt, erythritol, inositol , maltodextrin, mannitol, and sodium hydroxymethylcellulose are mixed uniformly; the above-mentioned mixture is uniformly mixed and dissolved by adding water to granulate with an extrusion granulator, then magnesium stearate is added, and the mixture is uniformly mixed again. drying and sieving; compressing the dried and sieved mixed granules to obtain buccal tablets; sterilizing the buccal tablets; sealing and packaging the sterilized buccal tablets. In the method herein, any device can be used as long as it can realize the above steps.
[0087] In addition, in the first step, high molecular weight hyaluronic acid or its salt, low molecular weight hyaluronic acid or its salt, ery...
Embodiment 1
[0092] (1) Raw material pretreatment
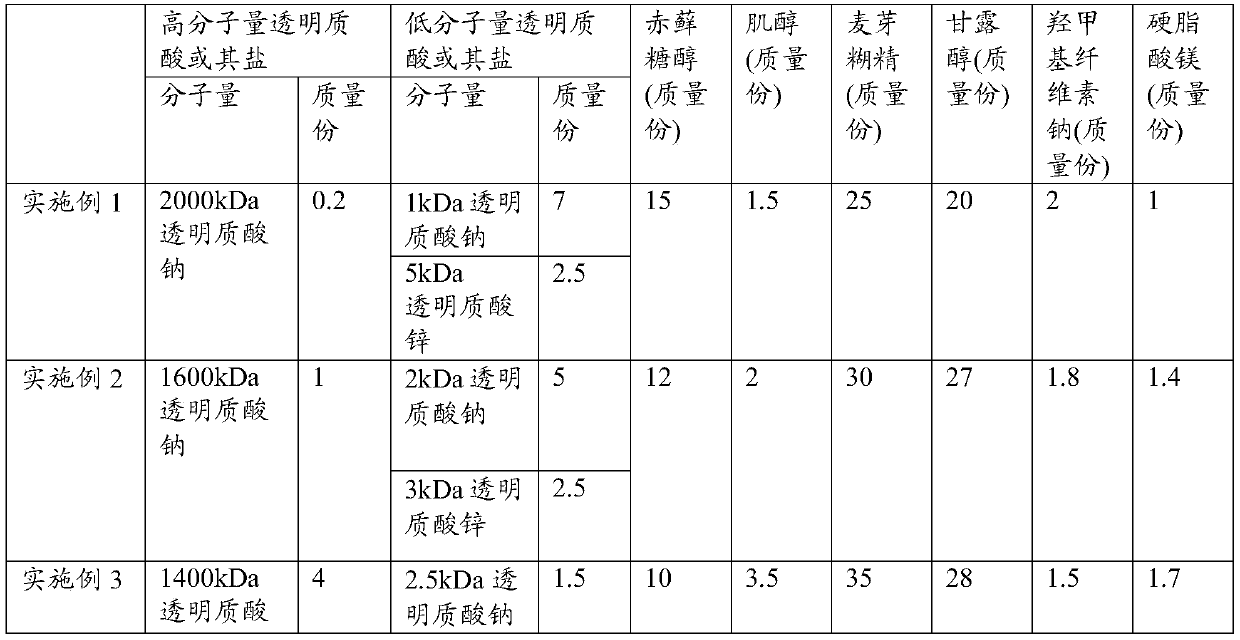
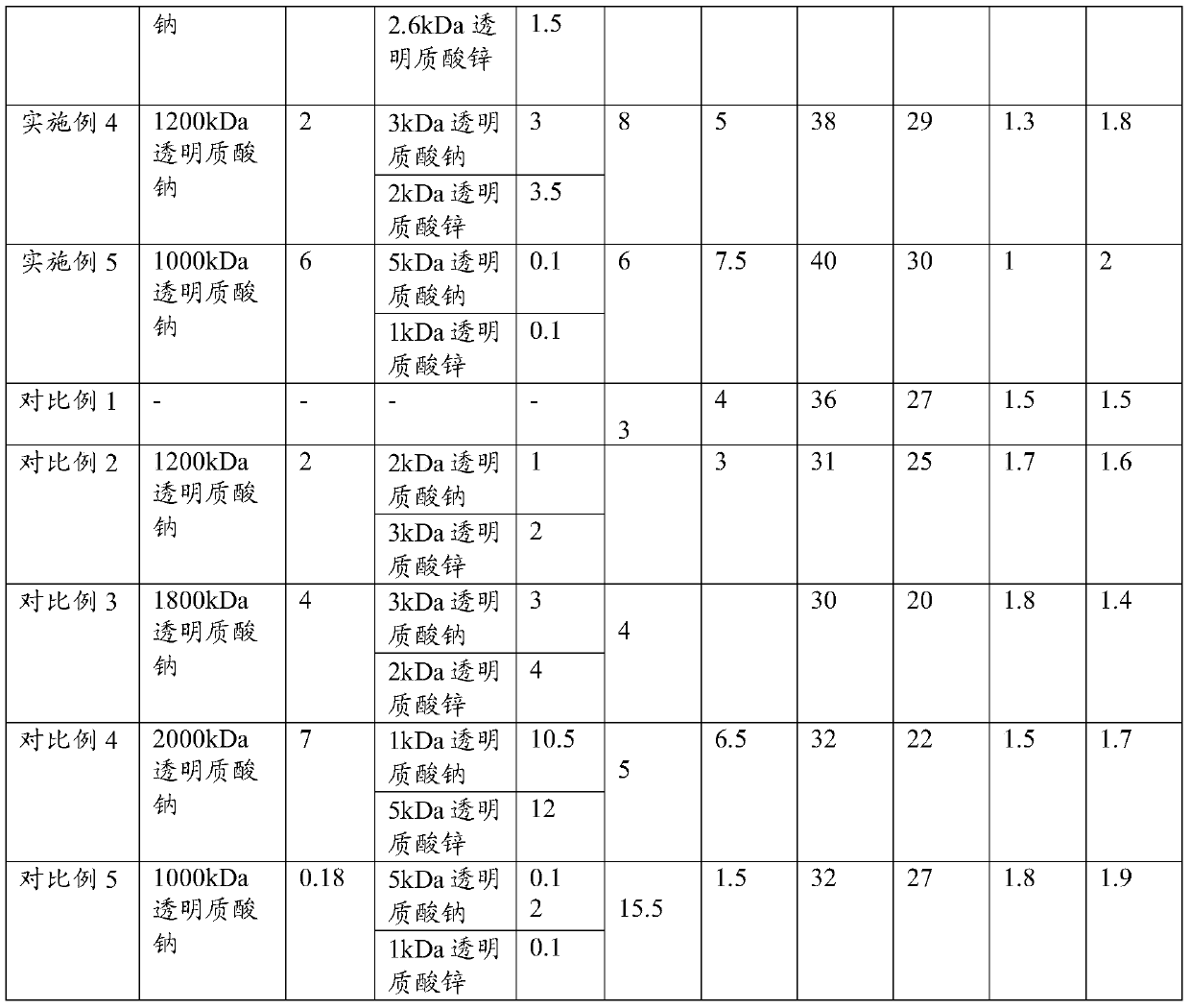
[0093] Take 0.2 parts of 2000kDa sodium hyaluronate, 7 parts of 1kDa sodium hyaluronate, 2.5 parts of 5kDa zinc hyaluronate, 15 parts of erythritol, 1.5 parts of inositol, 25 parts of maltodextrin, 20 parts of mannitol, hydroxymethyl Mix 2 parts of sodium cellulose base evenly.
[0094] (2) Granulation
[0095]Add 10g of purified water to the homogeneously mixed raw materials in step (1), use an extrusion granulator to granulate, dry in an oven at 40-50°C for 2 hours, pass through a 12-14 mesh sieve to crush large particles, and then pass through a 60 Mesh sieve to remove fine particles and fine powder;
[0096] (3) Tablet
[0097] Add 1 part of magnesium stearate to the above-mentioned granules, mix again uniformly to obtain a mixture, and perform tablet compression with an extrusion tablet machine, controlling the weight of a single tablet to 500 mg, and the resulting compressed tablet is named S1.
Embodiment 2
[0099] Take 1 part of 1600kDa sodium hyaluronate, 5 parts of 2kDa sodium hyaluronate, 2.5 parts of 3kDa zinc hyaluronate, 12 parts of erythritol, 2 parts of inositol, 30 parts of maltodextrin, 27 parts of mannitol, hydroxymethyl 1.8 parts of cellulose sodium were mixed evenly.
[0100] (2) Granulation
[0101] Add 10g of purified water to the homogeneously mixed raw materials in step (1), use an extrusion granulator to granulate, dry in an oven at 40-50°C for 2 hours, pass through a 12-14 mesh sieve to crush large particles, and then pass through a 60 Mesh sieve to remove fine particles and fine powder;
[0102] (3) Tablet
[0103] Add 1.4 parts of magnesium stearate to the above-mentioned granules, mix again uniformly to obtain a mixture, and compress the tablet with an extrusion tablet machine, and control the mass of a single tablet to 500 mg, and the resulting compressed tablet is named S2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com