Crystal form of linagliptin intermediate and preparation method thereof
An intermediate and crystal form technology, applied in the field of medicinal chemistry, can solve problems such as difficult removal, difficult filtration or centrifugal drying, and difficult purification, and achieve the advantages of simplifying separation and purification steps, reducing production and operating costs, and reducing waste liquid discharge. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
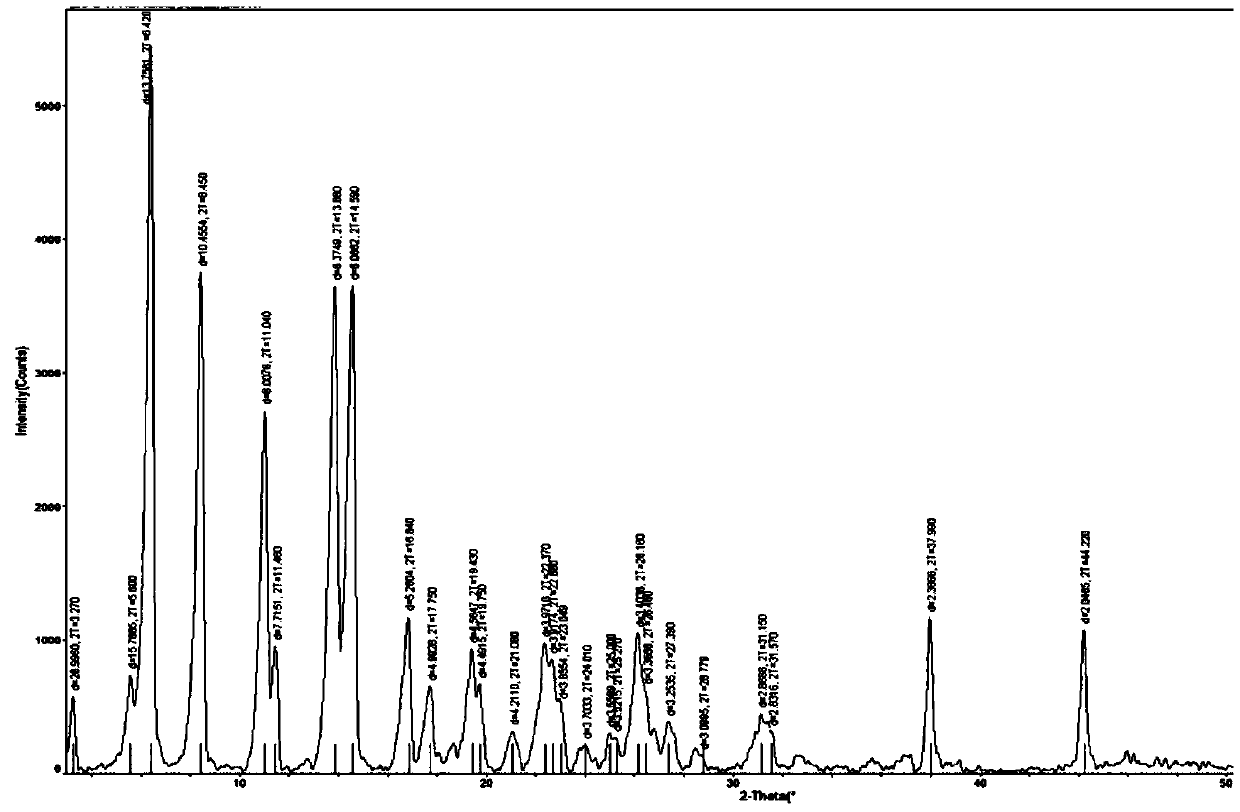
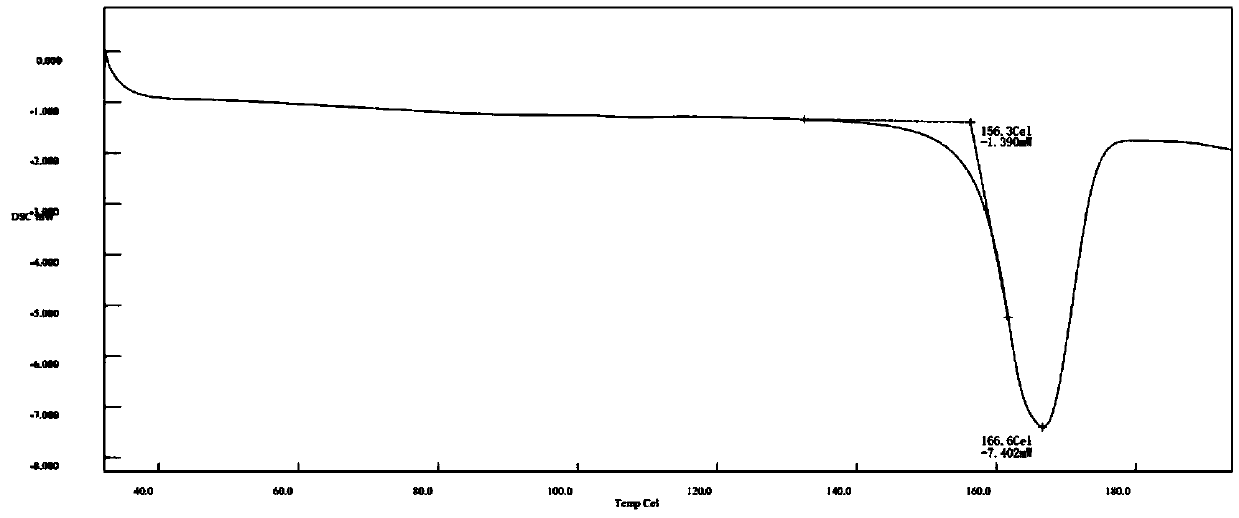
Image
Examples
Embodiment 1
[0044] At room temperature, add 8-bromo-7-(2-butynyl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazoline)methanol to the reaction flask base]-1H-purine-2,6-dione (100.00g, 220.6mmol), (R)-3-Boc-aminopiperidine (57.50g, 287mmol), potassium carbonate (122.00g, 883.0mol), acetonitrile 475ml, 25ml dimethyl sulfoxide (DMSO), stirred and heated to 84-86°C, kept stirring for about 20 hours, and the reaction was completed.
[0045] b) Add 750ml of purified water dropwise to the reaction solution in step a). After the dropwise addition, stir to dissolve, control the temperature at 65-67°C, keep stirring, and wait for white solid to precipitate, continue to keep warm for about 2 hours, slowly Cool down to about 25°C, stir for 1 hour, filter, and after the filter cake is drained, rinse and drain with 500ml of purified water to obtain a white crystalline powder solid product 1-[(4-methyl-quinazoline-2- Base) methyl] -3-methyl-7-(2-butyn-1-yl)-8-[(R)-3-(tert-butoxycarbonylamino)-piperidin-1-...
Embodiment 2
[0052] The preparation of tert-butoxycarbonyl linagliptin was carried out by adopting the steps of Example 1, the difference is that, in step b), the reaction liquid after adding water has a heat preservation crystallization temperature of 56-58°C. After steps a) to b), a white crystalline powder solid product D (120.31 g, 210.1 mmol) was obtained. The obtained white solid was in the form of crystal form A, with a yield of 95.23% and a purity of 99.877%.
[0053] Example 2(b)
[0054] The steps of Example 1 were used to prepare tert-butoxycarbonyl linagliptin, the difference being that, in step b), the reaction liquid after adding water had a heat preservation and crystallization temperature of 72-74°C. After steps a) to b), a white crystalline powder solid product D (120.29 g, 210.1 mmol) was obtained. The obtained white solid was in the form of crystal form A, with a yield of 95.22% and a purity of 99.930%.
Embodiment 3
[0056] The steps of Example 1 were used to prepare tert-butoxycarbonyl linagliptin, except that the solvent used in the reaction in step a) was 475ml of acetonitrile, 25ml of N,N-dimethylformamide, and the reaction time was 18 hours . After steps a) to b), a white crystalline powder solid product D (120.16 g, 209.8 mmol) was obtained. The obtained white solid was in the form of crystal form A, with a yield of 95.11% and a purity of 99.896%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com