Refined panax quinquefolius total saponin component and method for preparing panax quinquefolius total saponins through purification
A technology of American ginseng and total saponins, which is applied in the field of traditional Chinese medicine preparation technology, can solve the problems of difficult pretreatment, poor strength, and serious crushing, and achieve the effects of safe and controllable operating parameters, simple preparation process operation, and increased active ingredient content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] The present invention proposes a purification preparation method for extracting total saponins of American ginseng, the specific steps are as follows:
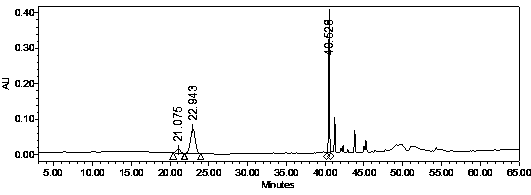
[0032] Weigh 100g of the pretreated American ginseng medicinal material, add 1L of 65% ethanol in a water bath, heat and reflux for extraction (90°C) for 3 hours, filter through filter paper, add 0.8L of 65% ethanol to the medicinal residues, and then heat and reflux for 2 hours in a water bath (90°C). Filtrate and combine the filtrates; concentrate by rotary evaporation and spray dry to obtain dry powder of the extract. The filtrate is carried out to HPLC analysis, and chromatographic analysis result is as follows: figure 1 shown.
[0033] Connect the C18 reverse-phase chromatographic column (filler weight 300g, particle size 60μm) to the high-pressure liquid phase system, design the flow rate 60ml / min (190cm / h), use 5% ethanol equilibrium buffer to wash the chromatographic column 3BV, and extract American ginseng L...
Embodiment 2
[0036] The present invention proposes a purification and preparation method for extracting total saponins of Panax ginseng, and the specific steps are as follows:
[0037]Weigh 100g of the pretreated American ginseng medicinal material, add 2L of 70% ethanol in a water bath, heat and reflux for extraction (90°C) for 2 hours, filter with gauze, add 1L of 70% ethanol to the medicinal residues, heat and reflux for extraction in a water bath (90°C) for 2 hours, and filter with gauze , the combined filtrates were concentrated by rotary evaporation and then spray-dried to obtain dry powder of the extract.
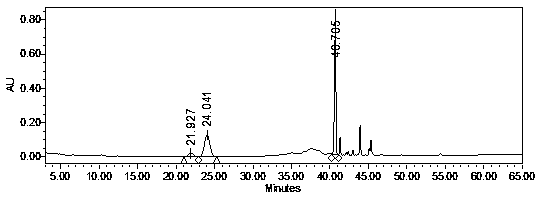
[0038] Connect the C18 reverse-phase chromatographic column (filler weight 300g, particle size 30μm) to the high-pressure liquid phase system, the design flow rate is 60ml / min (190cm / h), use pure water equilibrium buffer to wash the chromatographic column 3BV, and the American ginseng crude extract (Use a 0.22 μm filter membrane to remove particulate insolubles) Purify the chroma...
Embodiment 3
[0041] The present invention proposes a purification preparation method for extracting total saponins of American ginseng, the specific steps are as follows:
[0042] Weigh 100g of the pretreated American ginseng medicinal material, add 1.5L of 55% ethanol in a water bath, heat and reflux for extraction (90°C) for 2 hours, filter with gauze, add 1L of 55% ethanol to the medicinal residues, and then heat and reflux for extraction in a water bath (90°C) for 1 hour. Filtrate, combine the filtrates, concentrate by rotary evaporation, and then spray dry to obtain dry extract powder.
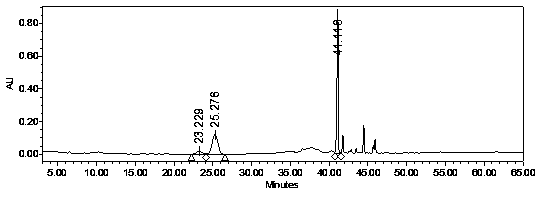
[0043] Connect the C18 reverse-phase chromatographic column (filler weight 300g, particle size 15μm) to the high-pressure liquid phase system, design the flow rate 50ml / min (150cm / h), use 10% ethanol equilibrium buffer to wash the chromatographic column 3BV, and extract American ginseng Liquid (use a 0.22μm filter membrane to remove particulate insoluble matter) through the system pump to flow through...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com