A method for efficiently preparing high-quality benzodifuranone
A high-quality technology for benzodifuranone, applied in the field of efficient preparation of high-quality benzodifuranone, can solve the problems of difficult removal of glacial acetic acid, long treatment cycle, long reaction cycle, etc., and achieve environmental protection and time-saving The effect of shortening the cycle time and reducing the production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
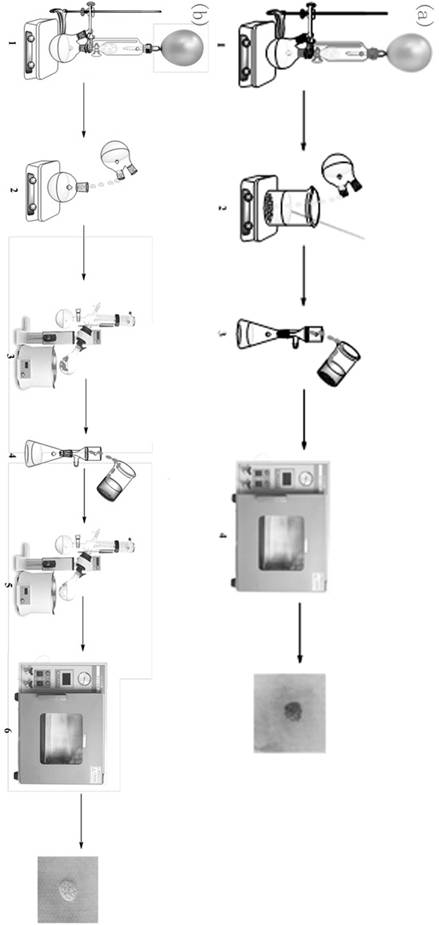
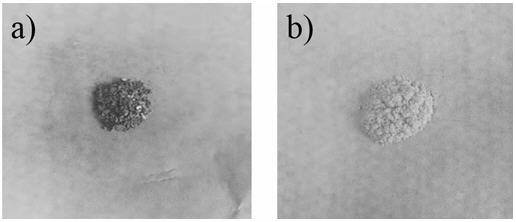
[0035] use Traditional Filtration The crude benzodifurandione was purified by filtration as figure 1 As shown in a:
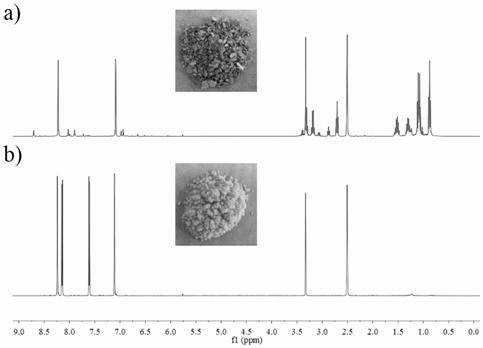
[0036] Place N,N-diethyl-furan-3-carboxamide (10g, 59.8mmol) and 40mL of anhydrous THF in a 100mL one-necked flask, and add 41.1mL of n-LiBu n-hexane solution (1.6M , n-LiBu 65.6mmol), after the dropwise addition was completed, the temperature was slowly raised to room temperature and stirred for 12.0 h. The mixture was poured into deionized water and stirred overnight at room temperature. Move the mixture into a sand core funnel (Φ80.5mm) equipped with filter paper (single layer-qualitative filter paper, medium speed 102 type, Φ80mm), filter, and wash the filter with 50mL deionized water, 50mL absolute ethanol, and 50mL petroleum ether in sequence. Cake, the filter cake was vacuum-dried at 50°C for 12 hours to obtain 1.14g of brown benzodifuranone, such as figure 2 Shown in a, the yield is 20.2%, and the purity is 65%, 1 H-NMR picture as image 3 As sh...
Embodiment 2
[0038] use Quartz sand / crude product / silica gel / filter paper The crude product of benzodifurandione is filtered and purified through the composite separation layer, such as figure 1 As shown in b:
[0039] Put N,N-diethyl-furan-3-carboxamide (8g, 47.8mmol) and 40mL of anhydrous THF in a 100mL one-necked flask, and add 32.5mL of n-LiBu n-hexane solution (1.6M , n-LiBu 52mmol), after the dropwise addition was completed, the temperature was slowly raised to room temperature and stirred for 5.0 h. The mixture was poured into deionized water and stirred at room temperature for 5.0 h. by rotary evaporator at 55 o C spin deionized water and THF, move the crude product into quartz sand (100 mesh, thickness 3mm) / crude product / silica gel (reagent grade, 300 mesh, thickness 25mm) / filter paper (single-layer-qualitative filter paper, medium speed 102 type, Φ80mm ) into the sand core funnel (Φ80.5mm) of the composite separation layer, washed with dichloromethane, 50mL each time, rinsed...
Embodiment 3
[0041] use Quartz sand / crude product / quartz sand / silica gel / filter paper Composite separation layer filtration purification of benzodifurandione crude product:
[0042] Put N,N-diethyl-furan-3-carboxamide (10g, 59.8mmol) and 50mL of anhydrous THF in a 100mL one-necked flask, and add 37.5mL of n-LiBu hexane solution (1.6M , n-LiBu 60mmol), after the dropwise addition was completed, the temperature was slowly raised to room temperature and stirred for 5.5 h. The mixture was poured into deionized water and stirred at room temperature for 6.0 h. Spin out deionized water and THF at 60°C with a rotary evaporator, and move the crude product into quartz sand (100 mesh, thickness 2mm) / crude product / quartz sand (100 mesh, thickness 3mm) / silica gel (reagent grade, 300 mesh, thickness 30mm) ) / filter paper (single layer-qualitative filter paper, medium speed 102 type, Φ80mm) in the sand core funnel (Φ80.5mm) of the composite separation layer, wash with dichloromethane, 50mL each time, r...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com