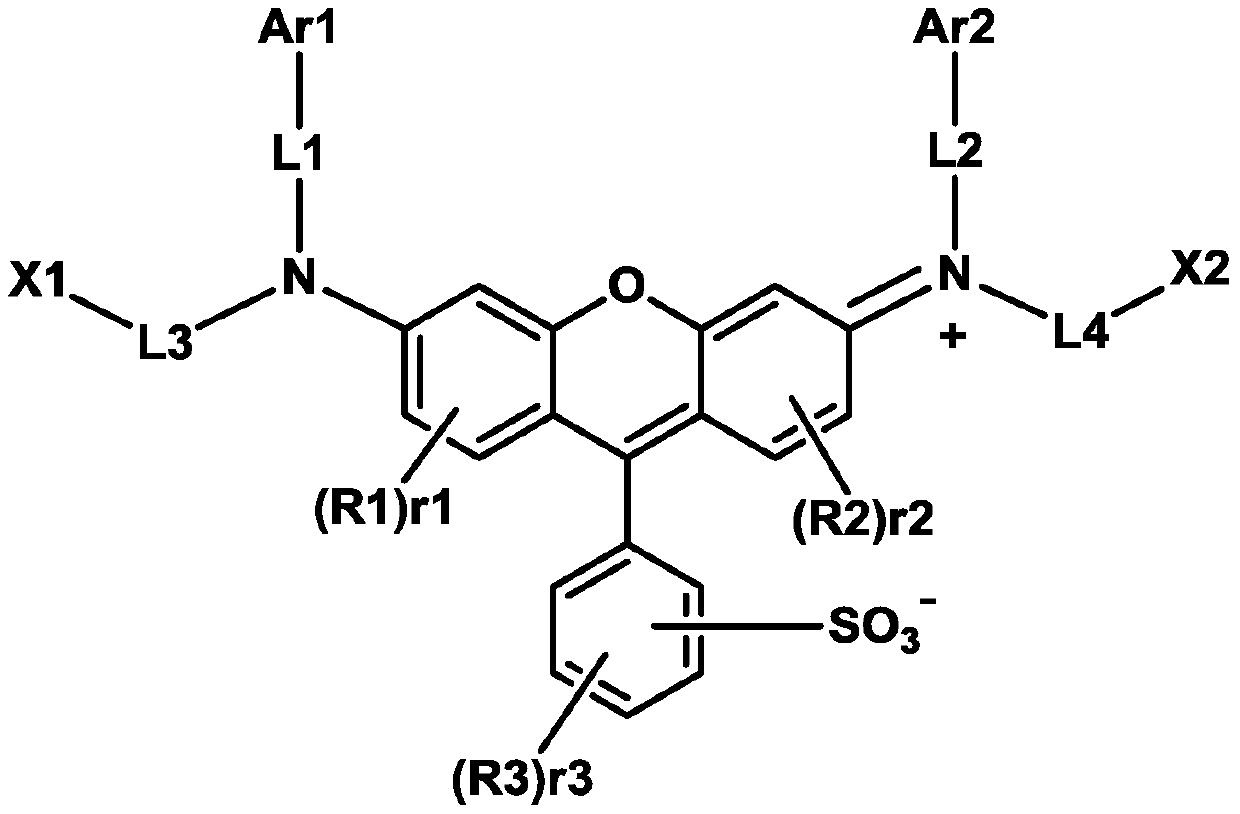
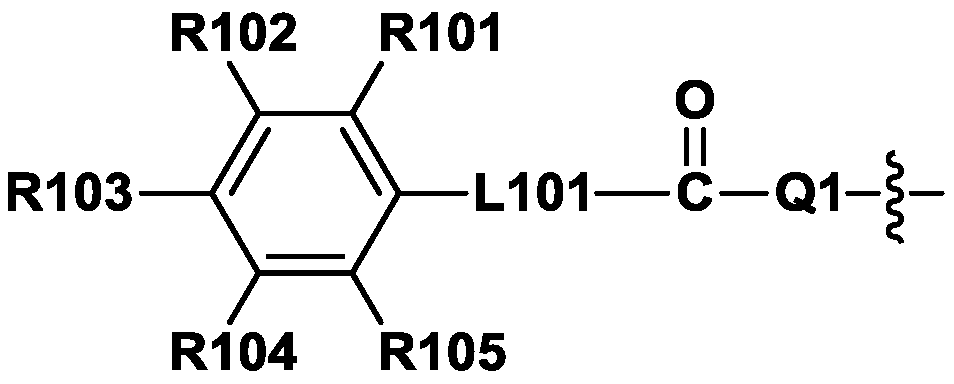
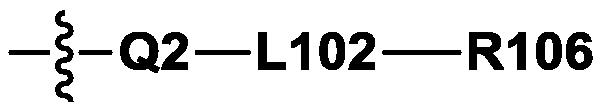Compound, polymer, colorant composition, resin composition, color filter, and display device
A compound and chemical formula technology, applied in the direction of instruments, optical filters, organic chemistry, etc., can solve the problems of low heat resistance and chemical resistance reduction, achieve excellent solubility, excellent heat resistance and chemical resistance, and save costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
manufacture example 1
[0255] -Manufacture of Compound 1-1
[0256] Production of [Compound A]
[0257]
[0258] Add compound A-1 (Benzenesulfonate Dichlorosulfofluorescein, benzenesulfonate dichlorosulfofluorescein) (3g, 7.40mmol, 1eq), compound A-2 (2-(Ethylamino)ethanol, 2 -(Ethylamino)ethanol) (5.28g, 59.22mmol, 8eq), deionized water (DI-water) 50g, and stirred at 100 degreeC. Then, the reaction was carried out overnight (12 hours). The reaction was terminated by quenching in 1M HCl solution (Solution), and NaCl (sodium chloride, sodium chloride) was added to precipitate the reactant. The generated precipitate was filtered under reduced pressure, and then dried in an oven (oven) at 80°C. After drying, in order to remove NaCl between the products, it was dissolved in DMF (Dimethylformamide, dimethylformamide) and filtered, and the filtrate was quenched (quenching) in diethyl ether, and the It was filtered and dried to obtain compound A (2.88 g, 5.64 mmol, 76%).
[0259] Ionization mode: A...
manufacture example 2
[0268] -Manufacture of Compound 1-4
[0269] Production of [Compound C]
[0270]
[0271] Add the compound A-4 (2.12g , 7.63mmol, 3eq), N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide hydrochloride (N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimidehydrochloride) (EDC-HCl ) (1.462g, 7.63mmol, 3eq), dimethylformamide (Dimethylformamide), 4-dimethylaminopyridine (4-Dimethyl aminopyridine) (DMAP) (0.244g, 2mmol), compound A (1.3g, 2.545 mmol, 1 eq) was produced by the same method as in Production Example 1-2 to obtain Compound C (1.15 g, 1.49 mmol) with a yield of 58.5%.
[0272] Ionization mode: APCI+: m / z=771[M+H]+, exact mass: 770
[0273] [Manufacture of compound 1-4]
[0274]
[0275] Add compound C (1.0g, 1.30mmol), D-MAP (0.487g, 2.0mmol), 2-hydroxyethyl methacrylate (2-Hydroxyethyl Methacrylate) (0.317ml, 2.6mmol), EDC (N-(3 -Dimethylaminopropyl)-N'-ethylcarbodiimide, N-(3-Dimethylaminopropyl)-N'-ethylcarbodiimide) (1.15g, 6.0mmol), by the same method as the product...
manufacture example 3
[0277] -Manufacture of Compound 1-6
[0278] Production of [Compound D]
[0279]
[0280] Add compound A-1 (Benzenesulfonate Dichlorosulfofluorescein, benzenesulfonate dichlorosulfofluorescein) (3g, 7.40mmol, 1eq) and diethanolamine (Diethanolamine) (3.89g, 37mmol, 5eq), by the same method as the production of compound A method to obtain compound D, 3.2 g (yield 79.8%).
[0281] Production of [Compound E]
[0282]
[0283] Add 3,5-di-tert-butyl-4-hydroxybenzoic acid (3,5-Di-tert-butyl-4-hydrobenzoic acid) of compound A-3 (2.54g, 10.16mmol, 4eq) and compound D (1.38 g, 2.54 mmol, 1 eq) was produced by the same method as the production of compound B to obtain 1.21 g of compound E (yield 47.3%).
[0284] Ionization mode: APCI+: m / z=1007[M+H]+, exact mass: 1006
[0285] Production of [Compound 1-6]
[0286]
[0287] Compound E (1.0g, 0.99mmol, 1eq) and 2-HydroxyethylMethacrylate (2-HydroxyethylMethacrylate) (0.36ml, 3mmol, 3eq) were added and produced by the same metho...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com