Glyphosate oxidase mutant, cloning, expression and applications thereof
A technology of glyphosate and oxidase, applied in the field of genetic engineering and molecular biology, can solve the problems of insufficient degradation performance of glyphosate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
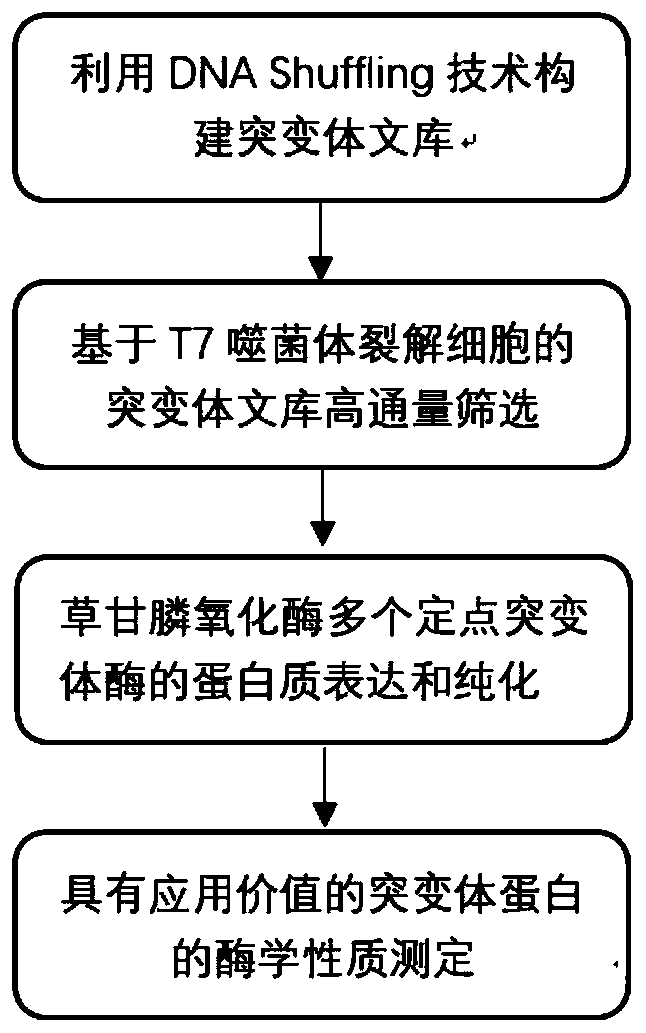
[0037] Example 1 Utilizes DNA shuffling technology to construct mutant library
[0038] In this example, the above-mentioned mutants B4S4, B4S6, B4S7, B4S9, and B4S11 were recombined to construct a library of glyphosate oxidase mutants, and the glyphosate oxidase mutant library was screened, and the affinity and catalytic efficiency for glyphosate were greatly improved. mutant.
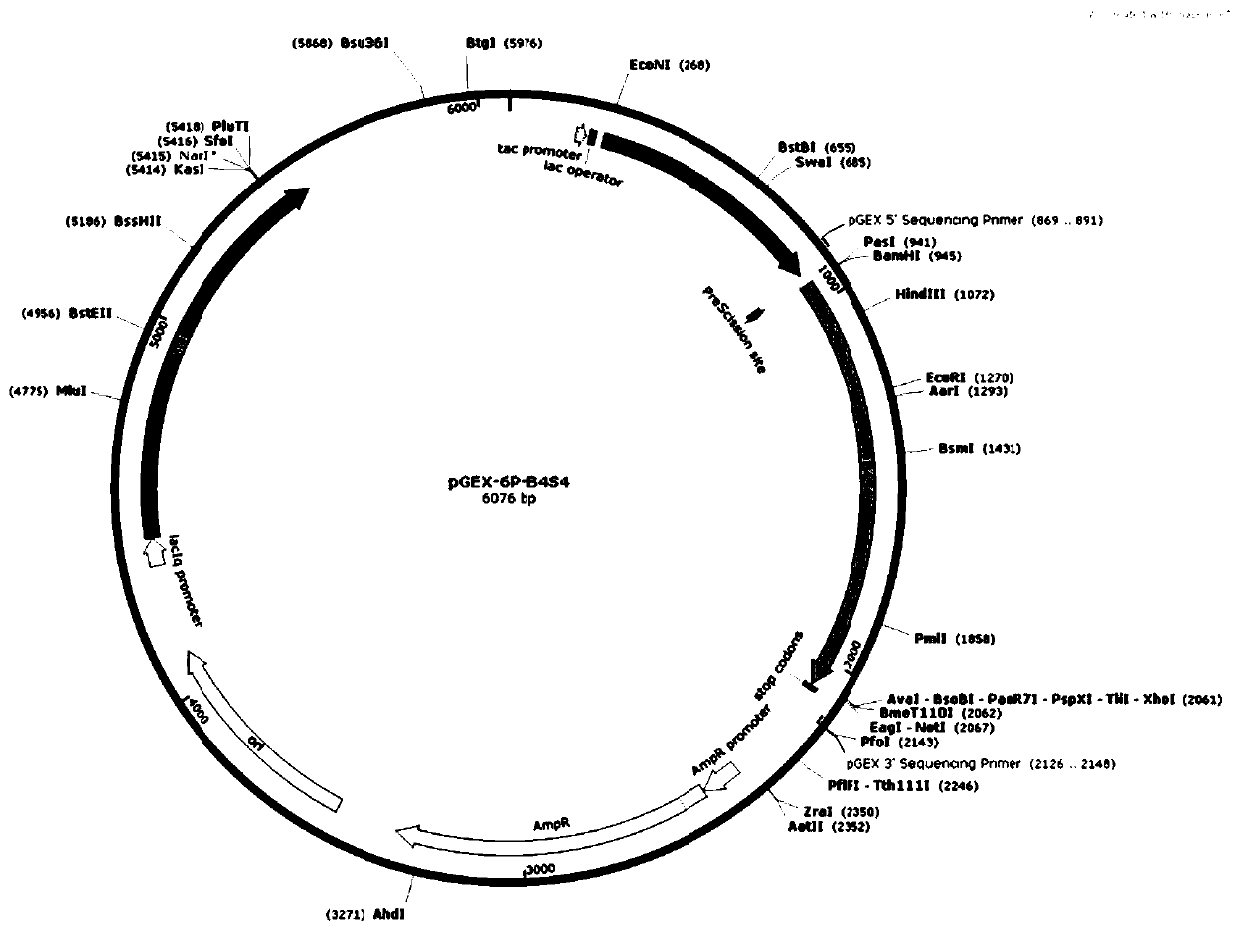
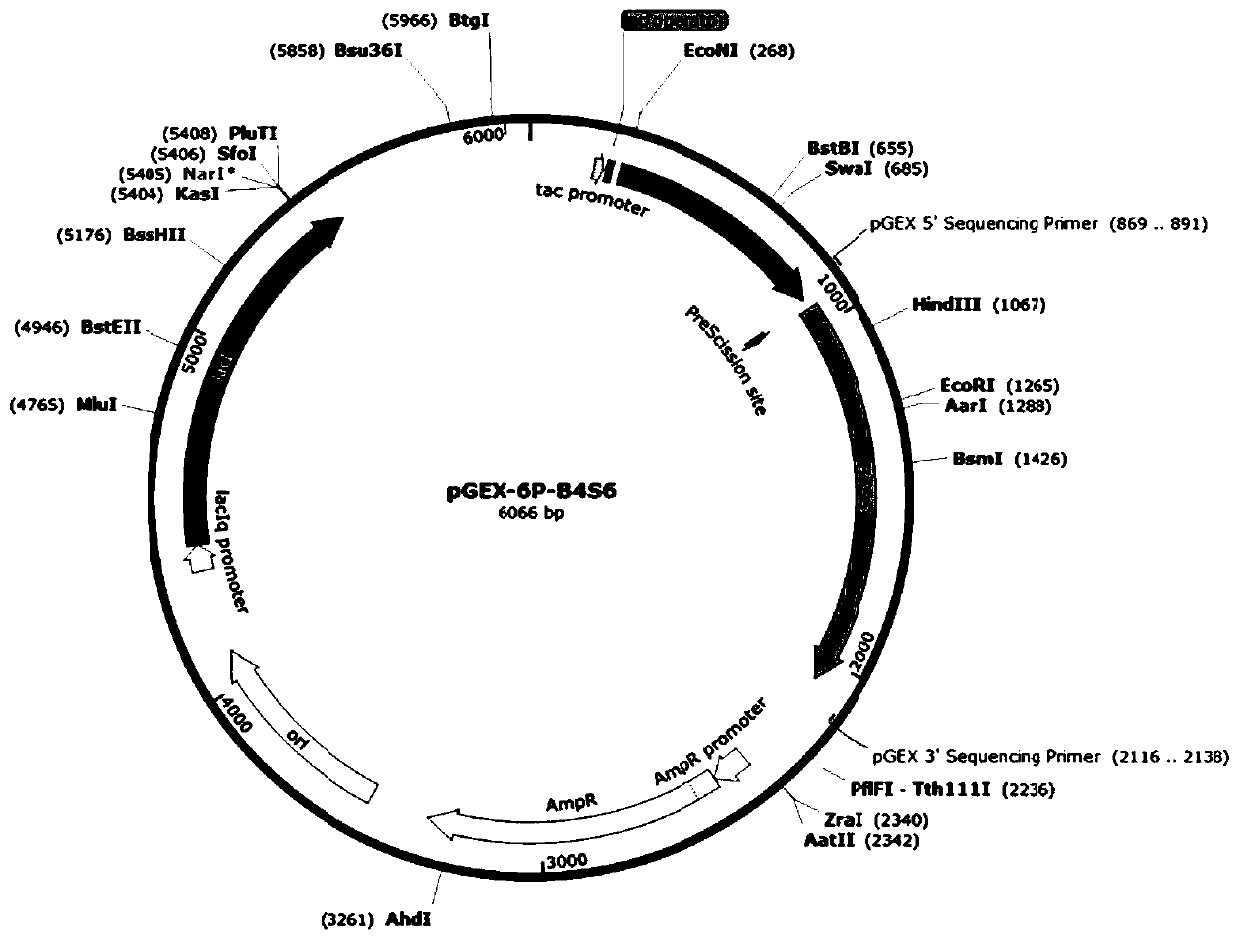
[0039] The above recombinant plasmids pGEX-6p-B4S4, pGEX-6P-B4S6, pGEX-6p-B4S7, pGEX-6p-B4S9 and pGEX-6p-B4S11 were used as mutation templates respectively (Yao, Lin et al.2015), refer to Stemmer report The DNA shuffling method and the use of ultrasonic method to process DNA fragments, DNA shuffling (Stemmer 1994, Miller, Pislaru et al. 2002), and the formed recombinant plasmid transformed Escherichia coli (E. Further improvement of Bacillus glycine oxidase to obtain glyphosate oxidase with better glyphosate degradation activity and form a mutant library.
[0040] (1) Amplification of the template g...
Embodiment 2
[0070] Screening of embodiment 2 mutants
[0071] The method in this example refers to the screening method reported by (Zhan, Zhang et al. 2013).
[0072] Use a sterilized toothpick to pick the control bacteria (Escherichia coli DH5α containing the recombinant plasmid pGEX-6p-B4S7) and the clones in the mutant library and inoculate them into 600 μL liquid LB medium (add ampicillin to a concentration of 100 μg / ml) in a 96-deep-well plate, and at the same time store the bacteria in each well on an ampicillin-resistant plate as a backup. Seal the 96-deep-well plate, shake and culture at 37°C and 200rpm for 16 hours, add LB solution containing ampicillin (100 μg / ml), IPTG (final concentration 0.1 mM) and T7 bacteriophage (Tarahovsky, Ivanitsky et al.1994) to each well 200 μL of culture medium, 28°C, 200 rpm shaking culture for 6 hours, so that the protein was induced to express and lysed by phage and released; pipette 159 μL of crude enzyme solution after lysis (the supernatant...
Embodiment 3
[0079] Embodiment 3 mutant protein expression and purification
[0080] Recombinant plasmid pGEX-6p-B5R18 containing mutant B5R18 and B5R26 genes (see attached Figure 8 shown), pGEX-6p-B5R26 (see attached Figure 9 shown) were mixed with E.coli BL21(DE3) competent cells, transformed by electric shock, spread on ampicillin-resistant plates (ampicillin concentration 100 μg / mL), and cultured overnight at 37°C until a single colony visible to the naked eye grew. Pick a single clone and inoculate it into 20 mL of liquid LB medium (containing 100 μg / mL of ampicillin), and culture overnight at 37° C. with shaking at 200 rpm. Transfer the bacterial solution to 2L of fresh liquid LB medium (containing ampicillin 100 μg / mL) according to the amount of 1%, and culture at 37°C and 200rpm to OD 600 was 0.6, added the inducer IPTG with a final concentration of 0.1 mM, induced and cultured for 12 hours at 22°C and 160 rpm to express the protein. The bacteria were collected by centrifugati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com