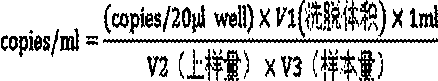EB virus detection kit based on droplet digital pcr
A micro-droplet and kit technology, applied in the biological field, can solve the problems of detection sensitivity and accuracy limitations, and achieve the effect of high sensitivity, accurate results, and accurate detection of virus content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] The Epstein-Barr virus detection kit based on droplet digital PCR of the present application includes:
[0044] (1) Reaction master mix for droplet digital PCR: including buffer (reaction buffer), dNTPs (deoxyribonucleoside triphosphate), taq enzyme (taq DNA polymerase), UDG enzyme (uracil-DNA glycosylation Enzymes); the droplet digital PCR reaction master mix should be compatible with the digital PCR equipment, and can be replaced with other digital PCR reaction master mixes, or can be prepared according to the digital PCR conditions.
[0045](2) Target fragment detection mixed solution and internal standard detection mixed solution: comprise the combination of primers and probes for detecting the target segment, and the combination of primers and probes for detecting the internal standard; the target segment is the DNA or DNA of Epstein-Barr virus in this embodiment Epstein-Barr virus DNA fragment (EBV DNA);
[0046] Wherein, the primer and probe combination of descr...
Embodiment 2
[0063] The use method of the Epstein-Barr virus detection kit based on droplet type digital PCR of the present application comprises:
[0064] (1). Sample collection: The applicable sample type is serum or plasma.
[0065] a. Serum Use a sterile syringe to draw 2ml of venous blood from the bend of the subject’s arm or the back of the hand. After the sample is separated from the serum, centrifuge at 1600rpm for 5 minutes at room temperature to separate the serum and transfer it to a 1.5ml sterilized centrifuge tube for later use.
[0066] b. Plasma Use a sterile syringe to draw 2ml of venous blood from the bend of the subject's arm or the back of the hand, inject it into a sterile collection tube containing EDTA, immediately mix it upside down gently, and centrifuge at 1600rpm at room temperature for 5 The plasma was separated in 1 minute and transferred to a 1.5ml sterilized centrifuge tube for later use.
[0067] Store samples immediately on ice or at 4°C after collection. ...
Embodiment 3
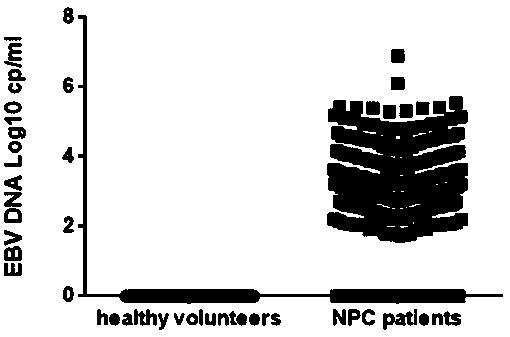
[0101] In this embodiment, the kit described in Embodiment 1 and the method described in Embodiment 2 are used to detect Epstein-Barr virus in the plasma samples of normal people and nasopharyngeal cancer patients. The specific detection methods are:
[0102](1). Sample extraction: MAGEN "HiPure AS Blood DNA Mini Kit" column extraction method was used to extract total DNA from plasma samples of 76 normal subjects and 268 newly diagnosed patients with nasopharyngeal carcinoma, positive control and negative control. According to the instructions of the nucleic acid extraction kit, 200ul samples were taken for extraction. When extracting, an internal standard was added to the samples, and the nucleic acid was extracted according to the operation instructions of the kit. The elution volume was 50µl.
[0103] (2). Reaction system preparation:
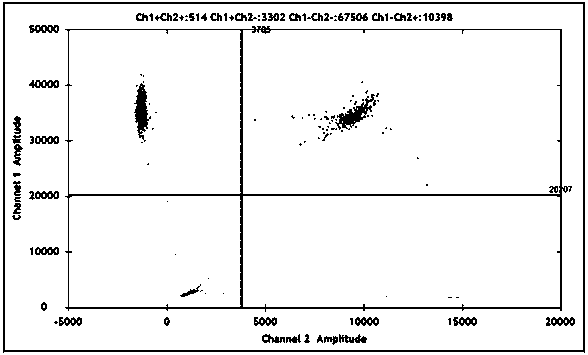
[0104] EBV DNA testing is carried out in batches, and each batch of testing needs to be tested in parallel with positive and negative contr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com