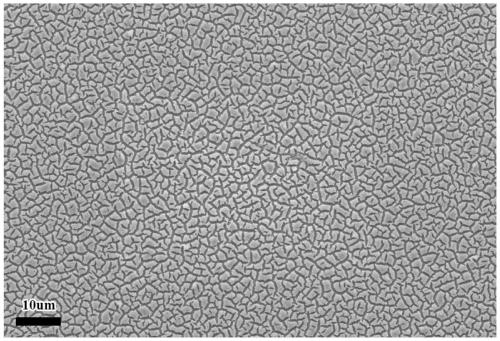
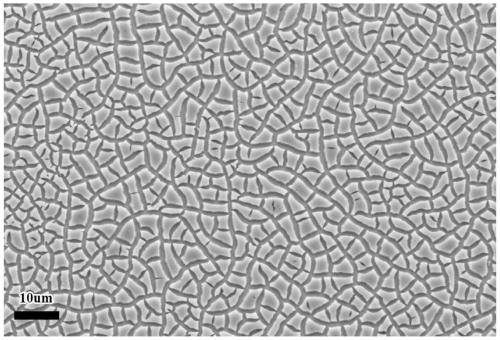
Non-noble metal amorphous water electrolysis anode material and in-situ growth preparation method thereof
A technology of anode material and non-precious metal, which is applied in the field of electrolyzed water anode material and its preparation, can solve the problems of reduced catalytic performance, reduced lifespan, and easy separation, and achieves low preparation cost, good conductivity, and is conducive to catalyzing water The effect of oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] an amorphous Ni 40 Fe 40 B 20 Water electrolysis anode material
[0057] 1), according to Ni 40 Fe 40 B 20 The nominal composition is to weigh Fe, Co, Ni, and B elements respectively. The mass percentage purity of Fe and Ni elements is not less than 99.95%, and the mass percentage purity of B element is not less than 99.9%;
[0058] 2), melting alloy ingot;
[0059] Put the weighed Fe, Ni, and B elements into a vacuum arc melting furnace for melting to obtain Ni 40 Fe 40 B 20 alloy ingot.
[0060] Melting parameters: before smelting, fill with 0.04Mpa mass percentage 99.999% argon as a protective atmosphere;
[0061] The required vacuum is 3.5×10 -3 Pa;
[0062] The melting current is 150A;
[0063] Melting time: 2-3 minutes for each smelting, 5-10 times of smelting;
[0064] 3), the single-roll quenching method prepares the amorphous strip;
[0065] The alloy ingot is mechanically broken into small pieces with a diameter of no more than 1cm, put into a q...
Embodiment 2
[0083] an amorphous Ni 60 Fe 20 B 20 Water electrolysis anode material
[0084] 1), according to Ni 60 Fe 20 B 20 The nominal composition is to weigh Fe, Ni, and B elements respectively. The mass percentage purity of Fe and Ni elements is not less than 99.95%, and the mass percentage purity of B element is not less than 99.9%;
[0085] 2), melting alloy ingot;
[0086] Put the weighed Fe, Ni, and B elements into a vacuum arc melting furnace for melting to obtain Ni 60 Fe 20 B 20 alloy ingot.
[0087] Melting parameters: before smelting, fill with 0.04Mpa mass percentage 99.999% argon as a protective atmosphere;
[0088] The required vacuum degree is 3.3×10 -3 Pa;
[0089] The melting current is 130A;
[0090] Melting time: 2-3 minutes for each smelting, 5-10 times of smelting;
[0091] 3), the single-roll quenching method prepares the amorphous strip;
[0092] The alloy ingot is mechanically broken into small pieces with a diameter of no more than 1cm, put into ...
Embodiment 3
[0110] An amorphous (NiFeCo) 80 B 20 Water electrolysis anode material
[0111] 1), according to (NiFeCo) 80 B 20 The nominal composition is to weigh Fe, Ni, Co, and B elements respectively. The mass percentage purity of Fe, Ni, and Co elements is not less than 99.95%, and the mass percentage purity of B element is not less than 99.9%;
[0112] 2), melting alloy ingot;
[0113] Put the weighed Fe, Ni, and B elements into a vacuum arc melting furnace for smelting to obtain (NiFeCo) 80 B 20 alloy ingot.
[0114] Melting parameters: before smelting, fill with 0.04Mpa mass percentage 99.999% argon as a protective atmosphere;
[0115] The required vacuum degree is 3.2×10 -3 Pa;
[0116] The melting current is 140A;
[0117] Melting time: 2-3 minutes for each smelting, 5-10 times of smelting;
[0118] 3), the single-roll quenching method prepares the amorphous strip;
[0119] The alloy ingot is mechanically broken into small pieces with a diameter of no more than 1 cm, p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com