Copper-nickel loaded TiO2 nanotube array electrode for reducing nitrate nitrogen in water
A nanotube array and nitrate nitrogen technology, applied in the field of electrochemistry, can solve the problems of long processing time, high current density, high cost, etc., and achieve good reduction effect and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
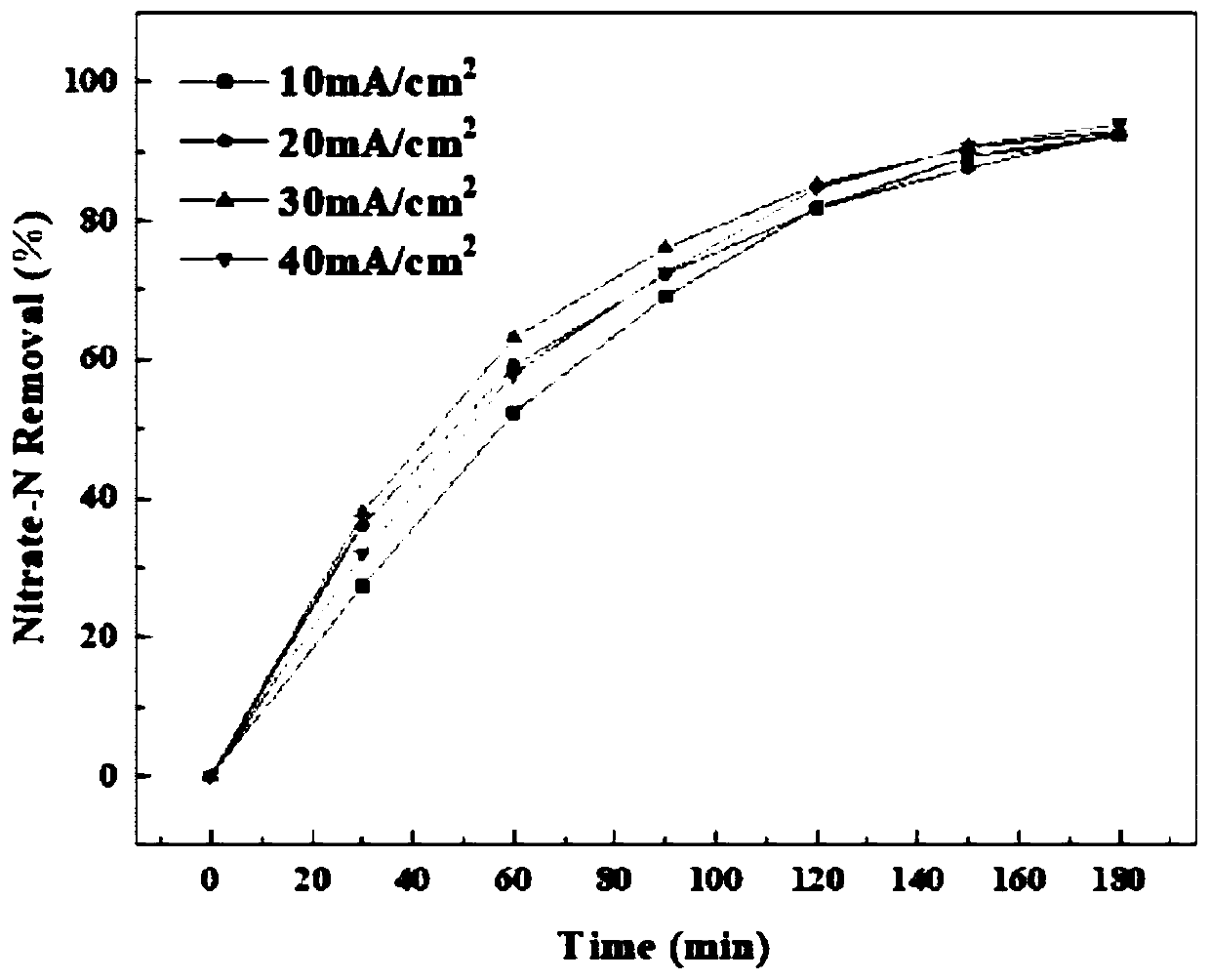
[0036] First, use 180 and 320 mesh sandpaper to polish the TC4 titanium plate with a size of 5cm×2.5cm, then etch it in a boiled 20wt% sulfuric acid solution for 10min, and finally place it in acetone and methanol for 15min and soak it with deionized water. Cleaning completes the pretreatment. After the titanium plate is dried, a layer of TiO is grown on the surface of the titanium plate through anodization treatment. 2 nanotube. The electrolyte used is an ethylene glycol solution with a mass fraction of 0.25% ammonium fluoride, the applied voltage is 45V, and the treatment time is 5h; after the material is dried, it is placed in a muffle furnace for calcination at 450°C for 1h. After the material is cooled to room temperature, electrodeposition treatment is carried out. The electrolyte is composed of a solution containing boric acid, copper sulfate, and nickel sulfate. The molar ratio is boric acid: copper sulfate: nickel sulfate == 13: 1: 8, the applied potential is consta...
Embodiment 2
[0038] Copper-nickel-loaded TiO 2 The preparation method of the nanotube array electrode is the same as that of Example 1. Embodiment 2 current density is 10mA / cm 2 , other conditions are with embodiment 1.
Embodiment 3
[0040] Copper-nickel-loaded TiO 2The preparation method of the nanotube array electrode is the same as that of Example 1. Embodiment 3 current density is 30mA / cm 2 , other conditions are with embodiment 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com