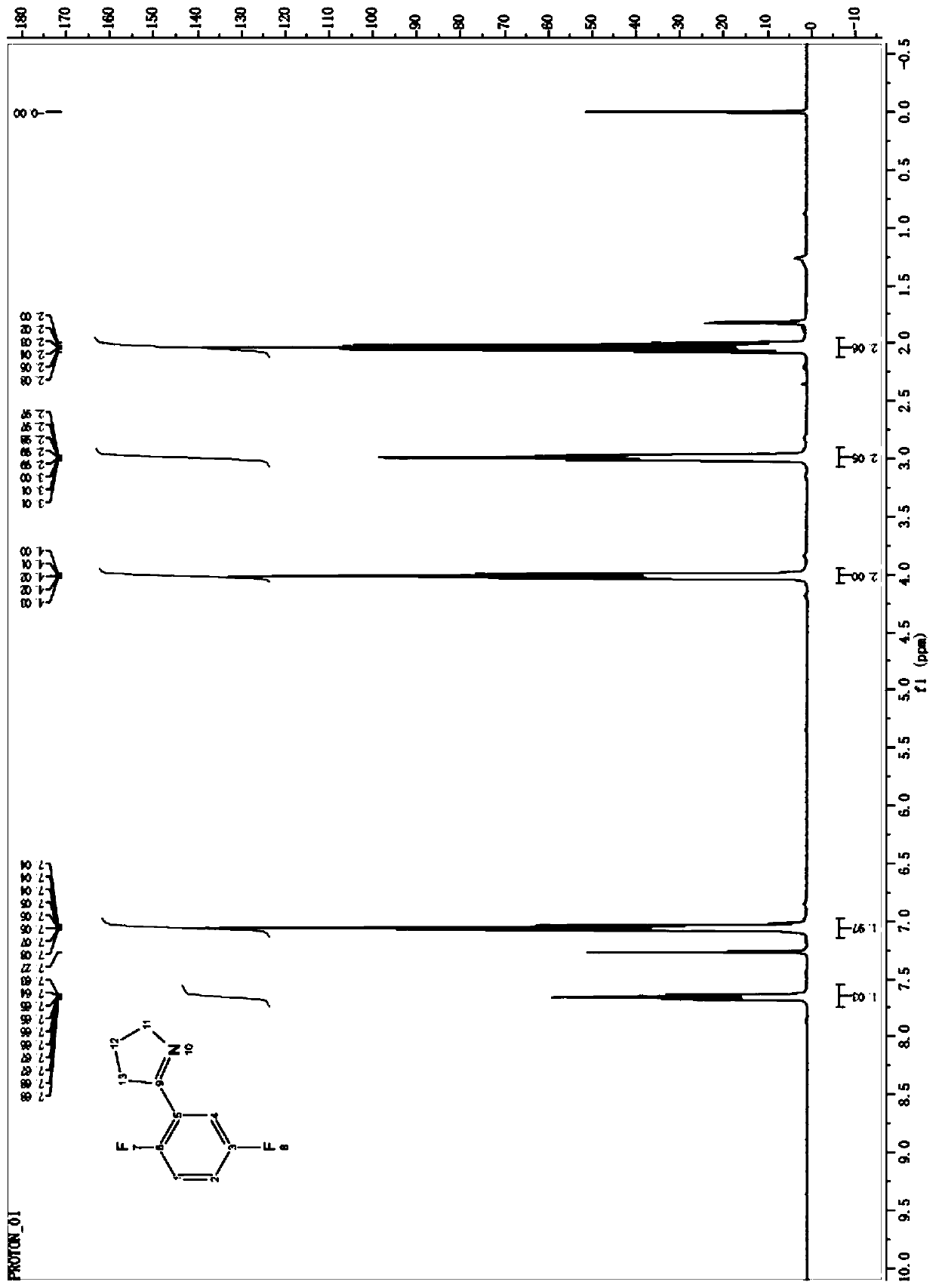
(R)-2-(2,5-difluorophenyl)pyrrolidine, preparation method and applications thereof
A difluorophenyl, pyrrolidine technology, applied in the field of biocatalysis, can solve the problems of high cost, serious pollution, poor stereoselectivity, etc., and achieve the effect of low cost and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] The preparation of embodiment 1 imine reductase
[0059] 1.1 Acquisition of imine reductase gene
[0060] According to the gene sequence SEQ ID NO.2, 4, 6, 8, 10 of the reported imine reductase SEQ ID NO.1, 3, 5, 7, 9, 11, 13, 15, 17, 19 encoded on NCBI , 12, 14, 16, 18, 20 whole gene synthesis imine reductase gene.
[0061] The information of each gene is shown in Table 1 below.
[0062] Table 1
[0063]
[0064]
[0065] 1.2 Preparation of imine reductase
[0066] Composition of LB liquid medium: peptone 10g / L, yeast powder 5g / L, NaCl 10g / L, dissolved in deionized water and then constant volume, sterilized at 121°C for 20min, ready for use.
[0067] The synthetic imine reductase gene was connected to the pET28a vector, and the enzyme-linked vector was transformed into the host E.coli BL21 (DE3) competent cells, and the engineered strain containing the IRED gene was obtained.
[0068] After the engineering bacteria containing the imine reductase gene were ac...
Embodiment 2
[0074] Example 2 Acquisition and expression of glucose dehydrogenase gene
[0075] The glucose dehydrogenase gene was fully synthesized according to the glucose dehydrogenase gene sequence derived from Bacillus subtilis 168 (NCBI accession number: NP_388275.1).
[0076] Glucose dehydrogenase gene is connected to pET28a, restriction site NdeI&HindIII, and the enzyme-linked vector is transformed into host E.coli BL21(DE3) competent cells to obtain an engineering strain containing glucose dehydrogenase gene. After the engineered bacteria containing the glucose dehydrogenase gene were activated by streaking on a plate, a single colony was picked and inoculated into 5 ml LB liquid medium containing 50 μg / ml kanamycin, and cultured with shaking at 37°C for 12 hours. Transfer to 50ml fresh LB liquid medium also containing 50μg / ml kanamycin according to 2v / v% inoculum amount, and shake to OD at 37°C 600 When it reaches about 0.8, add IPTG to its final concentration of 0.5mM, and indu...
Embodiment 3
[0077] The preparation of embodiment 3 glucose dehydrogenase enzyme crude enzyme liquid and enzyme activity assay
[0078] Wash 3 g of the bacteria collected in Example 2 twice with 0.1M pH 7.0 phosphate buffer, then resuspend the bacteria in 15 mL of pH 7.0 phosphate buffer, homogeneously break at low temperature and high pressure, and centrifuge the broken solution The precipitate is removed, and the obtained supernatant is a crude enzyme solution containing recombinant glucose dehydrogenase.
[0079] Enzyme activity detection method: the total reaction volume is 1mL. Take a cuvette with an optical path of 1.0 cm, add 880 μL of 100 mM phosphate buffer (pH 7.0) containing 400 mM glucose, and 100 μL of 25 mM NADP + Solution, placed in the colorimetric tank, turn on the heat preservation device on the ultraviolet spectrophotometer, 25, keep warm for 10min, dilute the prepared enzyme solution 200 times with deionized water, take 20μL and immediately add it to the colorimetric c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com