Time-resolved fluoroimmunoassay kit for detecting olaquindox and application thereof
A time-resolved fluorescence and immunoassay technology, used in analytical materials, biological tests, immunoglobulins, etc. Simple process and high detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
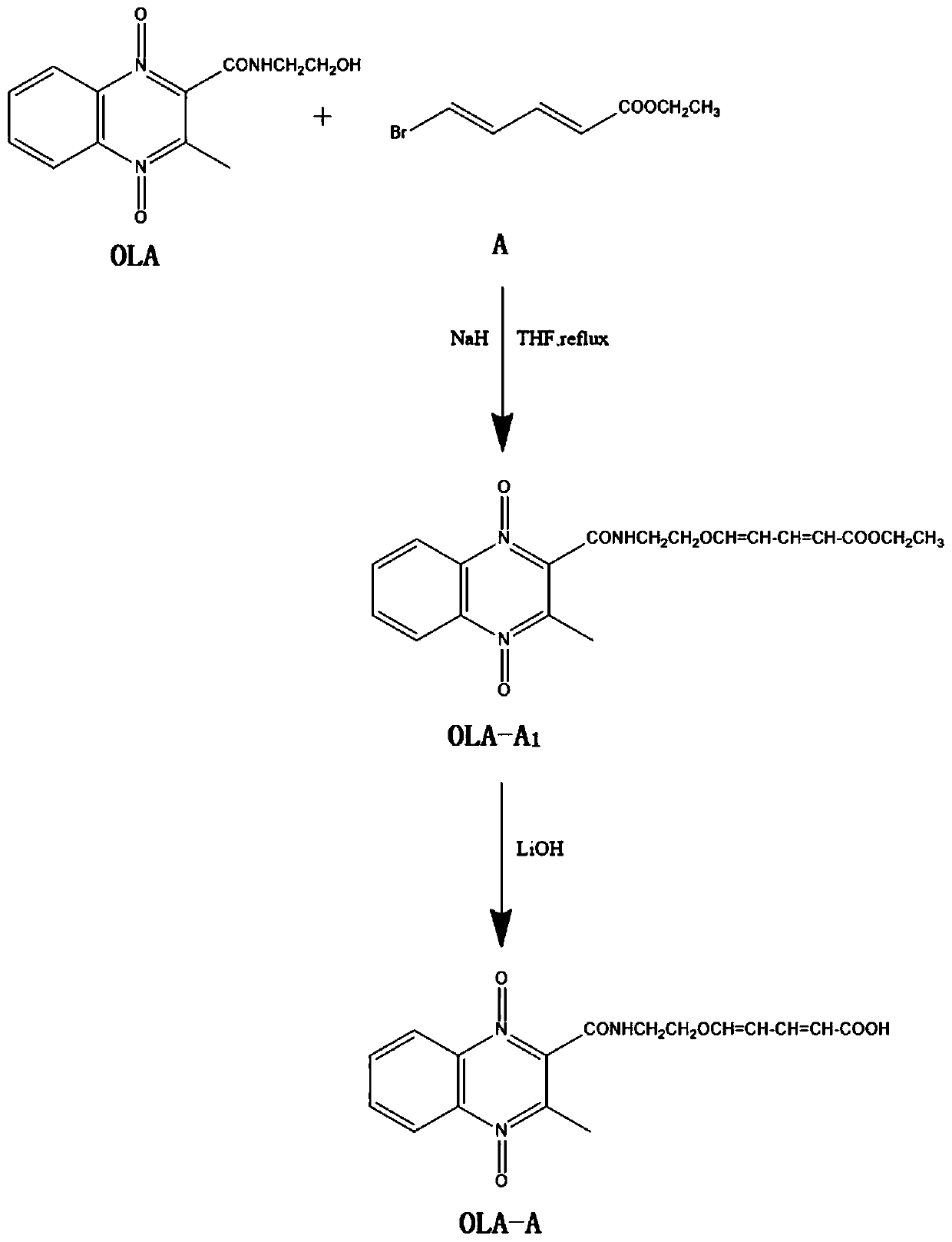
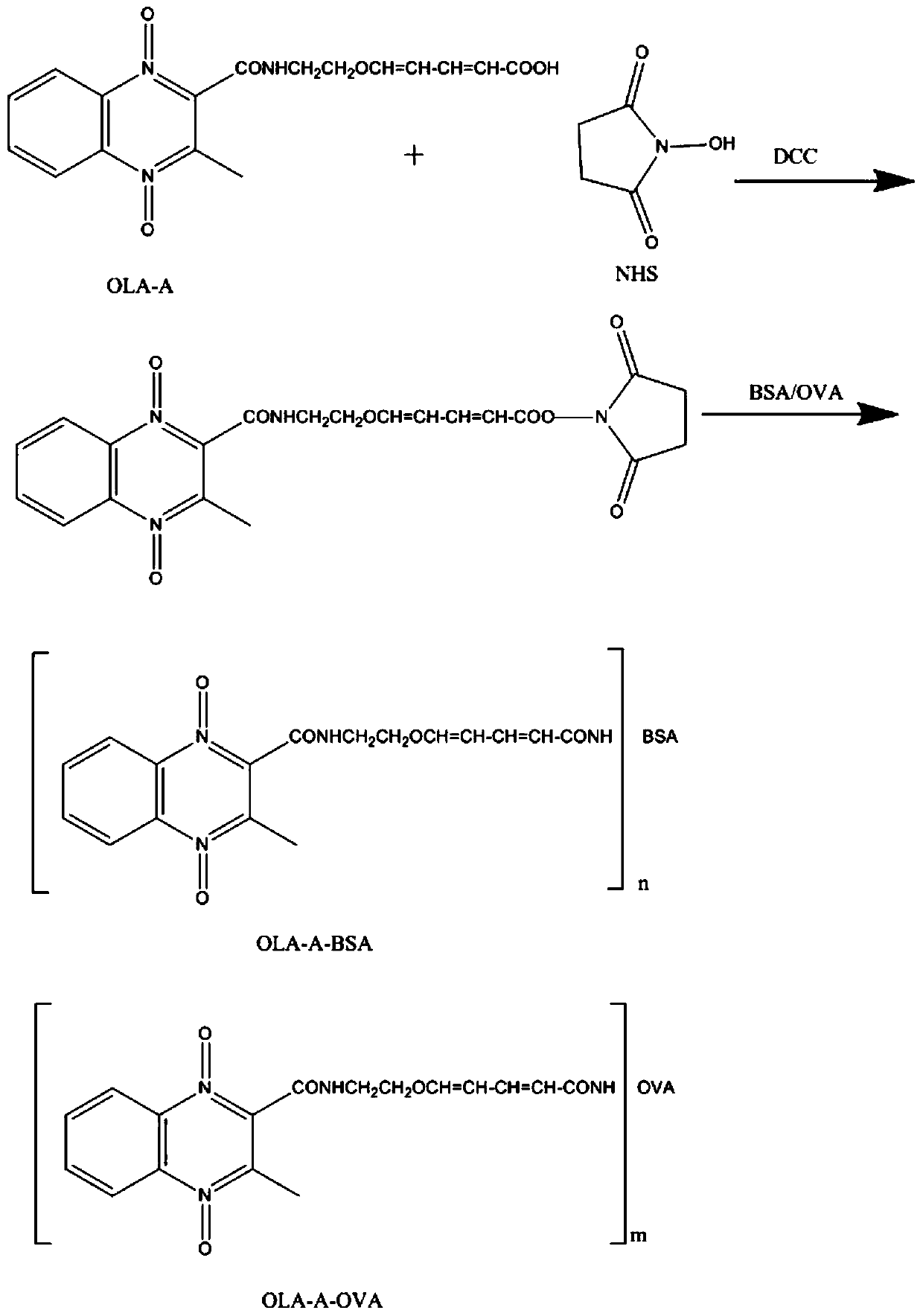
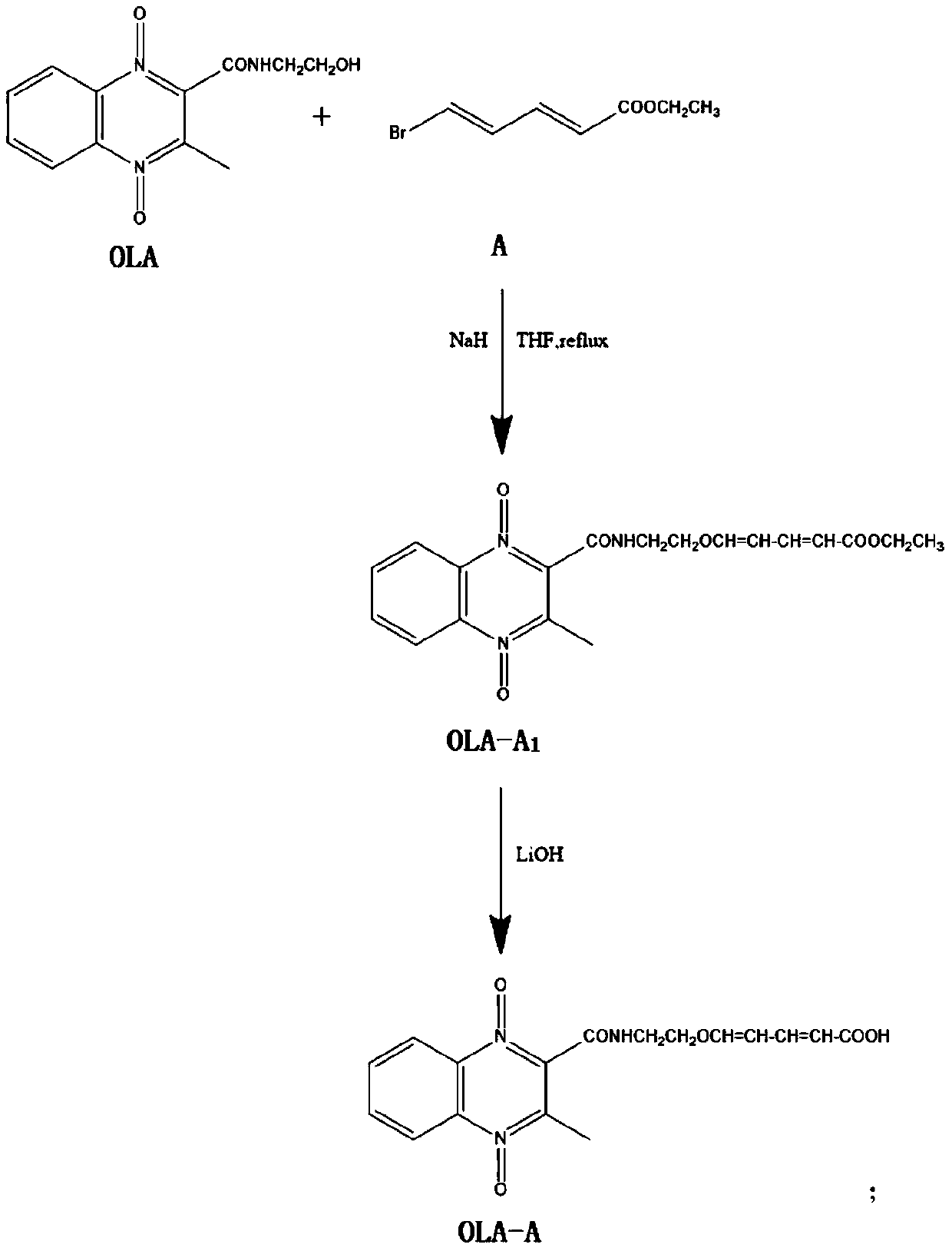
[0060] Synthesis of artificial antigen of olaquindox
[0061] (1) Synthesis of olaquindox hapten
[0062] (1.1) Dissolve 1mmol olaquindox in 2-5mL THF, add 1.1-1.2mmol NaH under ice bath (0°C), stir for 1 hour, then add 1mmol ethyl 5-bromo-2,4-dienevalerate (A), reflux reaction at 65°C for 6 hours, after the reaction is complete, add 30mL H 2 O, after extraction with ethyl acetate, combined organic phase, washed with saturated brine, rotary evaporation to remove solvent, separated by column chromatography to obtain OLA-A 1 , A 1 for -CH=CH-CH=CH-COOCH 2 CH 3 ; The eluent is: petroleum ether: ethyl acetate=1:1;
[0063] (1.2) Add 1mmol OLA-A 1 Dissolve in 10mL of methanol and water mixture (the volume of methanol and water are both 5mL), add 1.5mmol of lithium hydroxide, react at room temperature for 2h; after the reaction is complete, use 1mol / L hydrochloric acid solution to adjust the pH to 5-6 , extracted twice with 50 ml of ethyl acetate, the organic phase was washe...
Embodiment 2
[0087] Determination of antiserum titer:
[0088] The artificial antigens prepared in Example 1 and Comparative Example 1 were used to immunize BALB / C mice respectively. The artificial antigens were emulsified with complete Freund's adjuvant for the initial immunization. Booster immunization every day, a total of 3 booster immunizations, emulsified with incomplete adjuvant for booster immunization, immunological dose is 150 μg / mouse, after 14 days (days) after booster immunization, blood was collected from the tail of the mouse to measure the titer of multiple antiserum. The titer of antiserum was determined by ELISA method after doubling dilution with blocking solution, the mouse serum before immunization was used as negative control, and the OD of positive serum was used as 450nm Value and Negative Serum OD 450nm The dilution where the value ratio is greater than 2.1 is the antiserum titer, and the results are shown in Table 1. Finally, final immunization was carried out, ...
Embodiment 3
[0099] The preparation of embodiment 3 olaquindox monoclonal antibody
[0100] (1) Immunization of mice:
[0101] Select BALB / C female mice aged 6-8 weeks and weighing 18-20 g. Mix and emulsify the prepared immunogen (OLA-A-BSA) with an equal volume of complete Freund's adjuvant with a syringe, then inject at multiple points in the abdomen and underarms, with a dose of 100-200 μg per mouse, and then every 21 days Booster immunization was carried out, and blood was collected after 3 times of booster immunization to determine the titer. The titer was determined by indirect ELISA method. After the serum was diluted with blocking solution, the antiserum titer was determined by ELISA method. The mouse serum before immunization was used as a negative control. , with positive serum OD 450nm The dilution where the ratio of the value to the negative serum is greater than 2.1 is the antiserum titer. When the titer was no longer significantly increased, the dose was doubled for final ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com