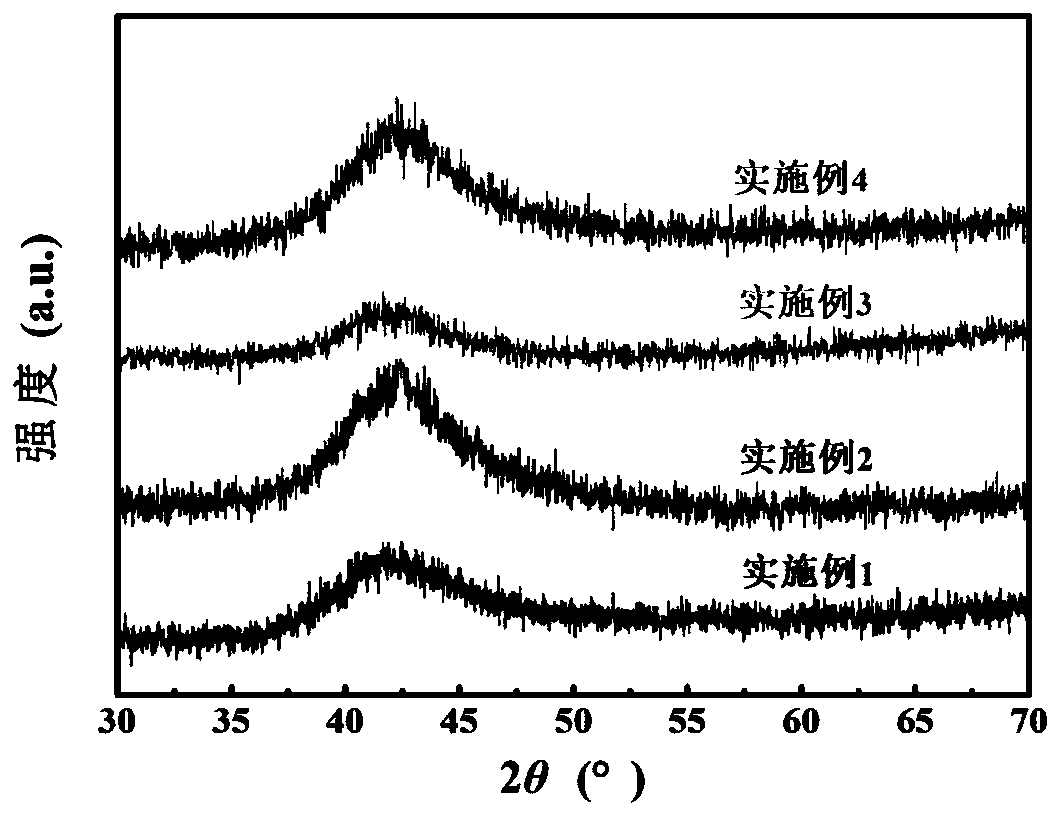
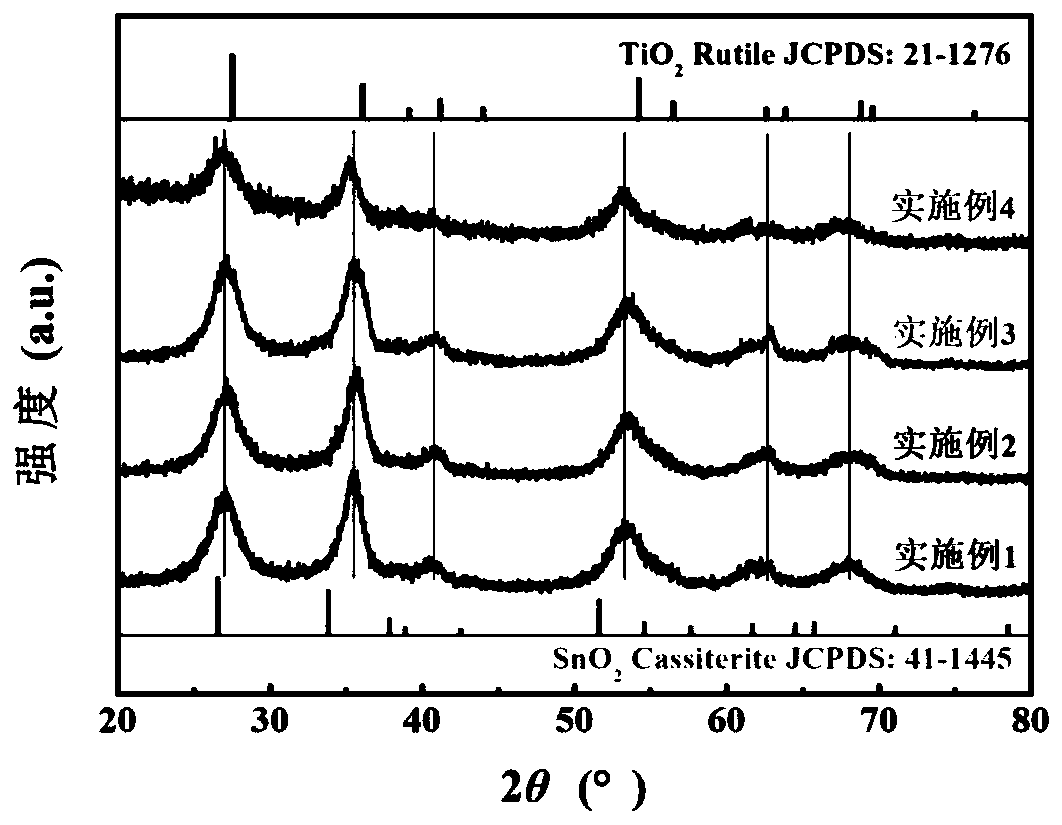
Preparation method of nano TiO2-SnO2 solid solution photocatalytic material
A photocatalytic material, solid solution technology, applied in catalyst activation/preparation, metal/metal oxide/metal hydroxide catalysts, chemical instruments and methods, etc. and other problems, to achieve the effect of shortening the preparation cycle, improving the corrosion activity, and delaying the corrosion effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Step 1: Select Cu, Ti, Sn, Y raw materials with a purity of 99.99wt%, carry out proportioning and weighing according to the atomic percentage Cu: 60%, Ti: 30%, Sn: 6%, Y: 4%, and then prepare the The metal raw materials and zirconium ingots used for deoxidation are placed in different stations of the vacuum electric arc furnace, and the vacuum is evacuated to 5.0×10 -3 Pa, and fill the furnace with argon with a purity of 99.9% as a protective atmosphere. During smelting, the current is adjusted in real time according to the melting state of the material, and the range is 100-200A. Note that the zirconium ingot is first melted to remove oxygen, the time is 40s, smelted once, and then the metal material is smelted, the single smelting time is 60s, smelted 5 times, and Cu 60 Ti 30 sn 6 Y 4 alloy ingot.
[0031] Step 2: Crush the alloy ingot obtained in step 1, take 5g and put it into a special quartz tube, then fix the quartz tube directly above the copper roller in t...
Embodiment 2
[0036] Step 1: Select Cu, Ti, Sn, Al raw materials with a purity of 99.99wt%, carry out proportioning and weighing according to the atomic percentage Cu: 60%, Ti: 30%, Sn: 6%, Al: 4%, and then prepare the The metal raw materials and zirconium ingots used for deoxidation are placed in different stations of the vacuum electric arc furnace, and the vacuum is evacuated to 5.0×10 -3 Pa, and fill the furnace with argon with a purity of 99.9% as a protective atmosphere. During smelting, the current is adjusted in real time according to the melting state of the material, and the range is 100-180A. Note that the zirconium ingot is first melted to remove oxygen, the time is 40s, smelted once, and then the metal material is smelted, the single smelting time is 55s, smelted 5 times, and Cu 60 Ti 30 sn 6 Al 4 alloy ingot.
[0037] Step 2: Crush the alloy ingot obtained in step 1, take 5g and put it into a special quartz tube, then fix the quartz tube directly above the copper roller i...
Embodiment 3
[0042] Step 1: Select Cu, Ti, Sn, In raw materials with a purity of 99.99wt%, carry out proportioning and weighing according to the atomic percentage Cu: 60%, Ti: 30%, Sn: 6%, In: 4%, and then prepare the The metal raw materials and zirconium ingots used for deoxidation are placed in different stations of the vacuum electric arc furnace, and the vacuum is evacuated to 5.0×10 -3 Pa, and fill the furnace with argon with a purity of 99.9% as a protective atmosphere. During smelting, the current is adjusted in real time according to the melting state of the material, and the range is 80-180A. Note that the zirconium ingot is first melted to remove oxygen, the time is 40s, smelted once, and then the metal material is smelted, the single smelting time is 50s, smelted 5 times, and Cu 60 Ti 30 sn 6 In 4 alloy ingot.
[0043] Step 2: Crush the alloy ingot obtained in step 1, take 5g and put it into a special quartz tube, then fix the quartz tube directly above the copper roller in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com