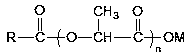
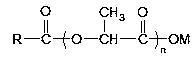
Preparation method of fatty acyl lactic acid or salt thereof
A technology of fatty acyl lactylate and fatty acyl lactylate, applied in the field of fine chemical preparation, can solve problems affecting product quality and downstream applications, and achieve the effect of high yield and low loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Put 220g of 90% lactic acid aqueous solution into the four-necked bottle, stir at 40°C, evacuate to a pressure of 2kPa, keep it for 2h, put in 284g of stearic acid, stir for 0.5h, put in a solid-phase composite catalyst formed by loading NaOH on 4A zeolite 4g. Raise the temperature to 200°C, react at a pressure of 10kPa for 2 hours, add NaOH to neutralize, recover the catalyst by filtration, lower the temperature, and discharge to obtain sodium stearoyl lactylate.
[0027] Simultaneously according to literature method: Geng Min et al. [Journal of Cereals and Oils of China, 2007 (06): 157-159] carry out comparative experiment, drop into 220g of 90% lactic acid in the four-necked bottle, add anhydrous sodium carbonate 21.2g to neutralize, stir until No bubbles were generated, heated to 235°C, put in 284g of stearic acid, stirred and reacted at constant temperature for 3h, lowered the temperature, and discharged to obtain a control sample.
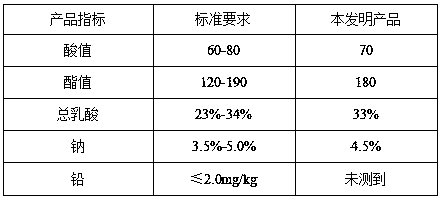
[0028] Measure the mass percen...
Embodiment 2
[0032] Preparation of solid catalyst: Weigh Na 2 CO 3 20g, add 30g of distilled water, stir to dissolve, add 80g of 4A zeolite, place on a constant temperature heating magnetic stirrer at 40°C for immersion and stirring for 2h, let stand and age for 1h, dry the water in an oven at 105°C, and then transfer to 500°C Dry in a muffle furnace for 2h, and cool to room temperature to obtain Na 2 CO 3 A solid-phase composite catalyst loaded on 4A zeolite.
[0033] Synthesis of sodium lauroyl lactylate: Put 180g of 50% lactic acid aqueous solution into a four-neck bottle, stir at 20°C, vacuumize to a pressure of 0.5kPa, keep for 0.5h, add 190g of lauric acid, stir for 1h, and put in 42g of the above catalyst. Raise the temperature to 140°C, react at a pressure of 10kPa for 4 hours, add NaOH aqueous solution to neutralize, the catalyst is recovered by filtration, the temperature is lowered, and the material is discharged to obtain an aqueous solution of sodium lauroyl lactylate. No...
Embodiment 3
[0036] Put 307kg of 88% lactic acid into the reaction kettle, stir at 30°C, evacuate to a pressure of 1kPa, keep it for 1h, put in 250kg of myristic acid, stir for 0.5h, put in CaCO 3 Load 52kg of solid-phase composite catalyst formed on 4A zeolite, raise the temperature to 190°C, react at a pressure of 20kPa for 6h, add Ca(OH) 2 Neutralize, recover the catalyst by filtration, cool down, and discharge to obtain the calcium myristoyl lactylate product.
[0037] Determine the content of myristic acid (calcium) in the product, and calculate the conversion rate to be 85.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com