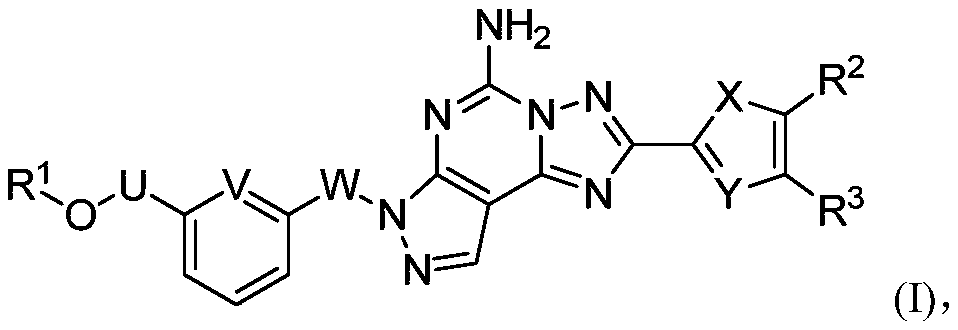
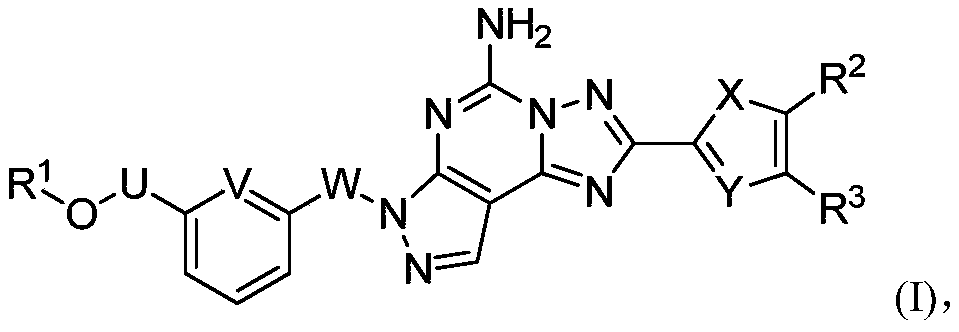
Nitrogen-containing fused tricyclic derivatives and application thereof
A compound, hydrate technology, applied in Parkinson's disease. , The field of nitrogen-containing fused tricyclic derivatives and pharmaceutical compositions containing these compounds can solve problems such as adenosine A distribution limitation, achieve good brain/plasma ratio, good clinical application prospects, and stable properties.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
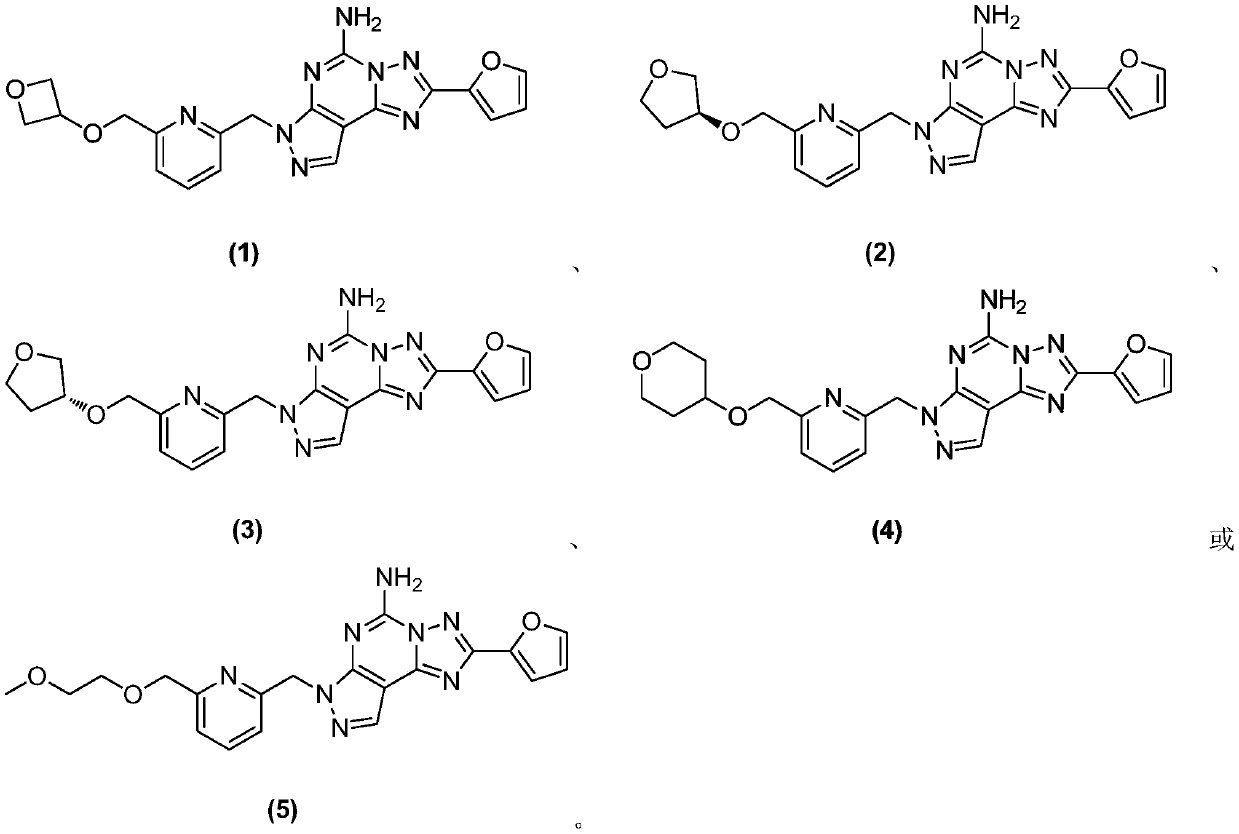
[0258] Example 1 2-(furan-2-yl)-7-((6-((oxetan-3-yloxy)methyl)pyridin-2-yl)methyl)-7H-pyrazole[ Synthesis of 4,3-e][1,2,4]triazol[1,5-c]pyrimidin-5-amine
[0259]
[0260] Step 1) Synthesis of 2-(bromomethyl)-6-((oxetan-3-yloxy)methyl)pyridine
[0261]
[0262] Add oxetan-3-ol (0.6g, 8.1mmol) and tetrahydrofuran (25mL) into a 100mL single-necked round-bottomed flask, stir at 5°C for 10 minutes, add sodium hydride (486mg, 12.2mmol), continue After stirring for 30 minutes, 2,6-bis(bromomethyl)pyridine (3.2 g, 12.1 mmol) was added, and transferred to an oil bath at 72° C. to react for 18 hours. Stop the reaction, add water (60mL) after cooling to room temperature, then add dichloromethane (60mL) for extraction, separate layers, collect the organic phase, spin dry under reduced pressure, separate and purify by column chromatography (petroleum ether / ethyl acetate (v / v)=3 / 1) The title compound was obtained as a light brown liquid (0.94 g, 45%).
[0263] MS(ESI,pos.ion)m / ...
Embodiment 2
[0269] Example 2 (S)-2-(furan-2-yl)-7-((6-(((tetrahydrofuran-3-yl)oxy)methyl)pyridin-2-yl)methyl)-7H- Synthesis of pyrazol[4,3-e][1,2,4]triazol[1,5-c]pyrimidin-5-amine
[0270]
[0271] Step 1) Synthesis of (S)-2-(bromomethyl)-6-(((tetrahydrofuran-3-yl)oxy)methyl)pyridine
[0272]
[0273] Add (S)-tetrahydrofuran-3-ol (0.9g, 10.22mmol) and tetrahydrofuran (35mL) into a 100mL single-necked round bottom flask, stir at 5°C for 10 minutes, then add sodium hydride (613mg, 15.3mmol), After continuing to stir for 30 minutes, 2,6-bis(bromomethyl)pyridine (4.0 g, 15.1 mmol) was added, and transferred to an oil bath at 75° C. for 8 hours. Stop the reaction, add water (60mL) after cooling to room temperature, then add dichloromethane (60mL) for extraction, separate layers, collect the organic phase, spin dry under reduced pressure, separate and purify by column chromatography (petroleum ether / ethyl acetate (v / v)=3 / 1) The title compound was obtained as a light brown liquid (1.1...
Embodiment 3
[0280] Example 3 (R)-2-(furan-2-yl)-7-((6-(((tetrahydrofuran-3-yl)oxy)methyl)pyridin-2-yl)methyl)-7H- Synthesis of pyrazol[4,3-e][1,2,4]triazol[1,5-c]pyrimidin-5-amine
[0281]
[0282] Step 1) Synthesis of (R)-2-(bromomethyl)-6-(((tetrahydrofuran-3-yl)oxy)methyl)pyridine
[0283]
[0284] Add (R)-tetrahydrofuran-3-ol (0.7g, 7.9mmol) and tetrahydrofuran (30mL) into a 100mL single-necked round bottom flask, stir at 5°C for 10 minutes, then add sodium hydride (477mg, 11.9mmol), After continuing to stir for 30 minutes, 2,6-bis(bromomethyl)pyridine (3.16 g, 11.9 mmol) was added, and transferred to an oil bath at 72° C. for 18 hours. Stop the reaction, add water (60mL) after cooling to room temperature, then add dichloromethane (60mL) for extraction, separate layers, collect the organic phase, spin dry under reduced pressure, separate and purify by column chromatography (petroleum ether / ethyl acetate (v / v)=3 / 1) The title compound was obtained as a light brown liquid (1.0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com