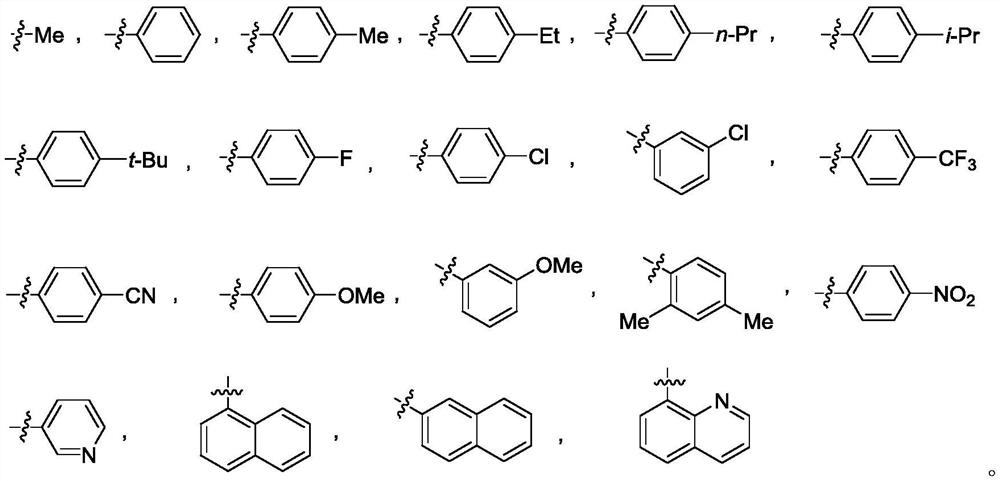
Compound and its use in the synthesis of brivaracetam intermediates and raw materials
A compound and reducing agent technology, applied in the fields of sulfonyl-substituted compounds and their synthesis, synthesis of brivaracetam intermediates and raw materials, can solve the problem of being unsuitable for large-scale industrial production, unable to be purified by crystallization or requiring column chromatography , purification and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104] Embodiment 1 prepares compound II
[0105] Add compound I (5.1 mmol) and 30 mL methanol into a 100 mL three-neck flask with mechanical stirring function, and stir at room temperature. Add sodium methoxide (1.10 g, 20.4 mmol) in batches, raise the temperature to 35-40° C., and stir for 10 minutes. The temperature was lowered to 20-25°C, and R-epichlorohydrin (0.71 g, 0.97 mmol) was added, and the addition was completed in about 10 minutes. The internal temperature rose to 50-55°C and stirred for 4h. Add 5ml of water and 5ml of acetic acid mixed solvent, and stir for 15h. The reaction solution was extracted by adding 20 mL of water and 30 mL of dichloromethane, the aqueous phase was extracted with dichloromethane (30 ml*2), and the organic phases were combined. Wash twice with saturated sodium bicarbonate solution (15 mL*2), dry the organic phase with anhydrous sodium sulfate, remove most of the solvent under reduced pressure, ethanol crystallization or column chromato...
Embodiment 2
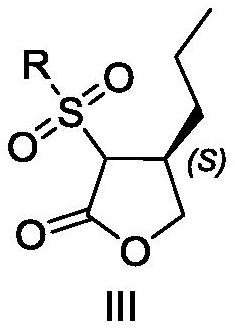
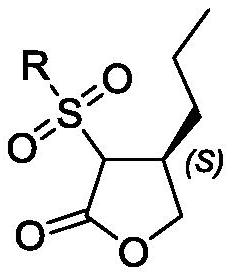
[0111] Embodiment 2 prepares compound III
[0112] Add tetrahydrofuran (10ml) and cuprous iodide (224mg, 1.18mmol) into a 100mL three-necked flask with mechanical stirring function, cool to -45°C-50°C, add ethylmagnesium chloride (2.1ml, 4.2mmol) dropwise, about 1h dropwise addition is complete. Afterwards, the temperature was raised to -5°C and stirring was continued for 1 h, then a solution of compound II (1.49 mmol) in tetrahydrofuran (5 ml) was added dropwise, and the temperature was maintained at -5 to -10°C, and the addition was completed in 45 min. After 15 min, saturated ammonium chloride aqueous solution (5 ml) was slowly added, followed by MTBE (5 ml), and stirred for 2.5 h. Stand to separate and separate, collect the organic phase, extract the aqueous phase with 10ml MTBE again, combine the organic phases, wash with water (10ml*2), wash with saturated brine (10ml), dry over anhydrous sodium sulfate, filter with suction, concentrate, add an appropriate volume mixed...
Embodiment 7III-3
[0126] The preparation of embodiment 7III-3
[0127] Add 100ml of tetrahydrofuran and cuprous cyanide (10.6g, 117.8mmol) into a 2.0L three-necked flask with mechanical stirring function, cool to -45°C-50°C, add ethylmagnesium bromide (185ml, 370.0mmol) dropwise, About 1h dropwise completed. Stirring was continued for 1 h, and a tetrahydrofuran solution of compound II-3 (42.2 g dissolved in 240 ml tetrahydrofuran) was added dropwise. The temperature was maintained at -45° C. to 50° C., and the addition was completed in about 1 h. Warm up to -15°C and stir for 2h, add saturated aqueous ammonium chloride solution (400ml), then add ethyl acetate (400ml), stir for 2h. Stand to separate layers, collect the organic phase, extract the water phase with 200ml ethyl acetate once more, combine the organic phases, wash with water (200ml*2), wash with saturated brine (200ml), dry over anhydrous sodium sulfate, filter with suction, concentrate, add Isopropanol was crystallized to obtain 23...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com