Porous boron nitride loaded iron nano material as well as preparation method and application thereof
A technology of boron nitride and iron nanometers, which is applied in the fields of chemical instruments and methods, chemical/physical processes, oxidized water/sewage treatment, etc., can solve the problems of limited catalytic effect, achieve removal benefits, large specific surface area, high ratio The effect of surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Embodiment 1: a kind of preparation method of porous boron nitride supported iron nanomaterial, specifically comprises the following steps:
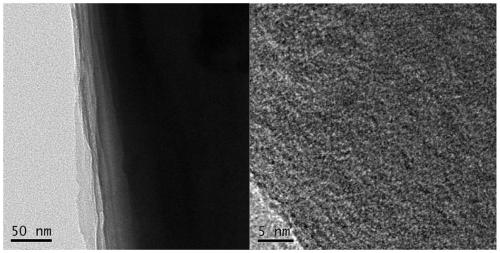
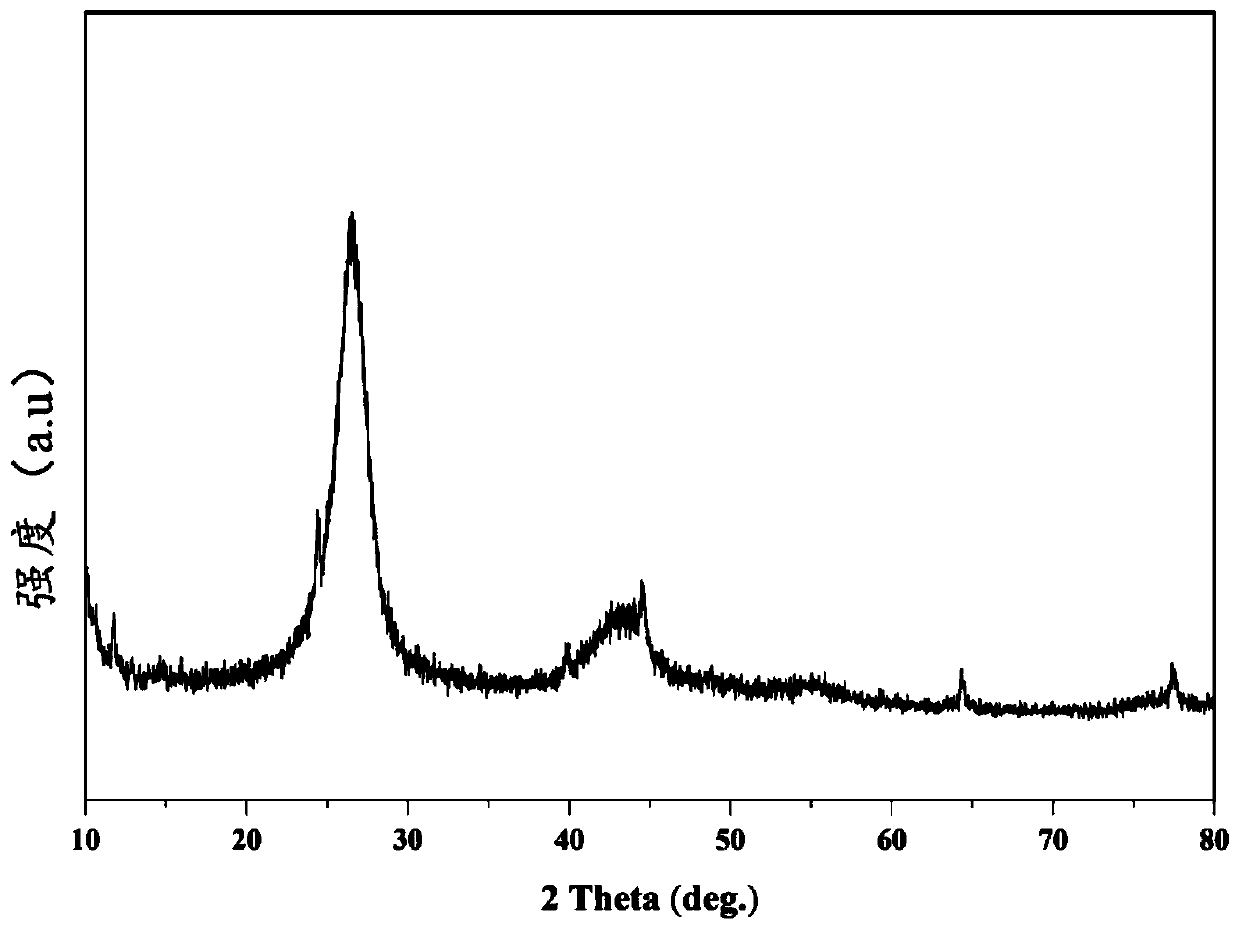
[0041]Weigh 5.5074g melamine and 5.3762g boric acid into 500mL ultrapure water, heat to 95°C and stir to dissolve. Dry after cooling, and then grind to get the powder, pour the powder obtained above into the quartz cup, put it into the tube furnace after covering it, and put it in the NH 3 The temperature was raised to 1500° C. for 4 h in the air at 5° C. / min for calcination, and then cooled naturally to obtain porous boron nitride (PBN). Weigh 3.5841g Fe(NO 3 ) 3 9H 2 O and 10.9312g PBN were added to a 500mL beaker, and 200mL ultrapure water was added to stir at 85°C for 4h. The sand core funnel was suction filtered (washed three times with ethanol and ultrapure water), and the obtained product was ground to obtain the final product Fe-PNB (porous boron nitride-supported iron nanomaterial). The prepared porous boron nitride-...
Embodiment 2
[0044] Embodiment 2: Various advanced oxidation processes are compared to the oxidation efficiency control experiment of oxytetracycline hydrochloride. The specific process and results are as follows:
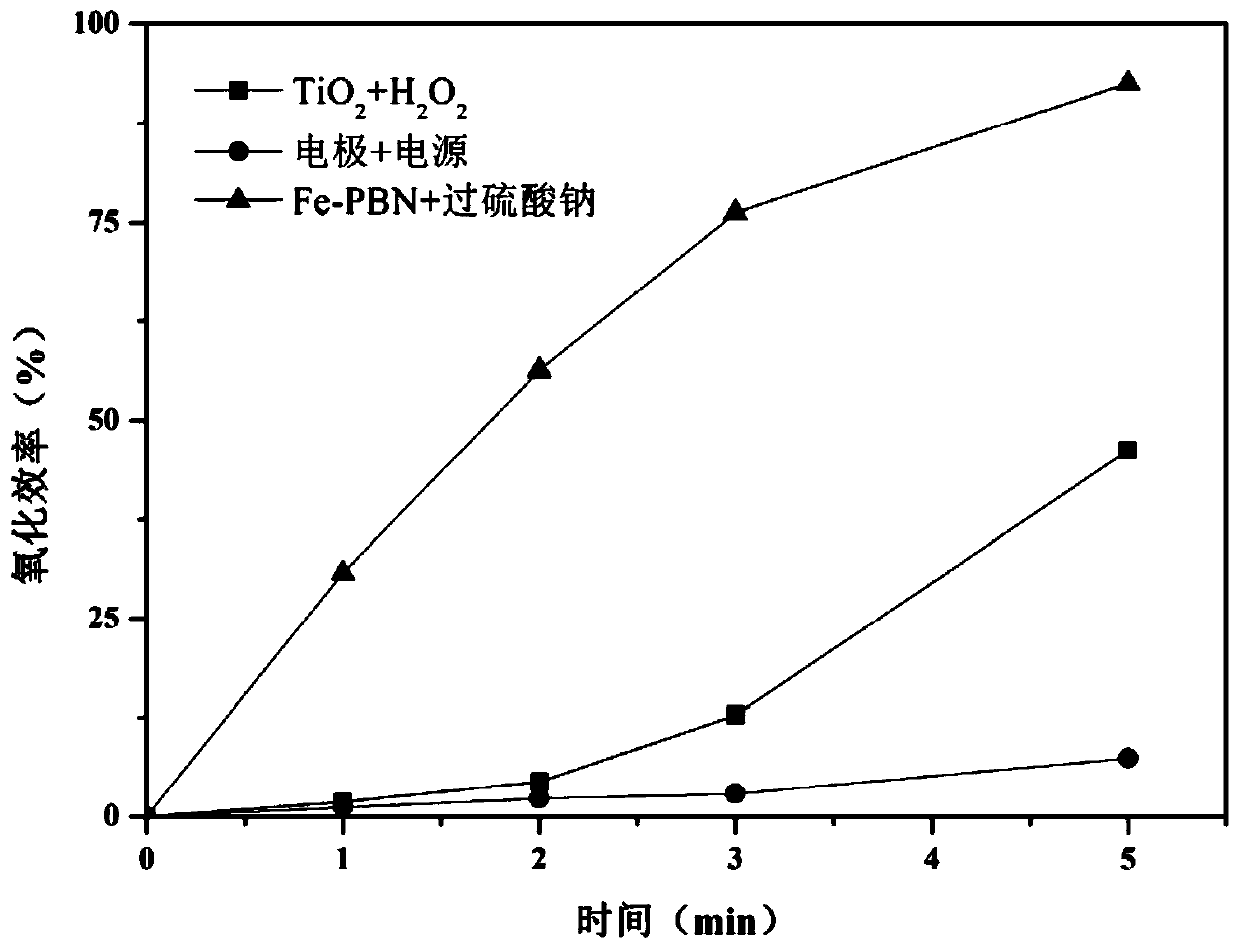
[0045] In order to discover the advantages of the combination of porous boron nitride-supported iron nanomaterials and sodium persulfate, we added photocatalytic (TiO 2 and H 2 o 2 ) and electrocatalysis (electrode + power supply) control experiments. Configure 0.04mmol / L oxytetracycline hydrochloride solution for TiO 2 / H 2 o 2 (20mg / L, 4mmol / L), iron electrode / power supply, Fe-PNB / PMS (100, 150mg / L) experiments, the results are as follows image 3 As shown, after 5min of reaction, TiO 2 and H 2 o 2 The oxidation efficiency of oxytetracycline hydrochloride is 45.2%; the oxidation efficiency of electrode + power supply to oxytetracycline hydrochloride is 6.4%; the oxidation efficiency of porous boron nitride loaded iron nanomaterials and sodium persulfate to oxytetracyc...
Embodiment 3
[0046] Embodiment 3: Porous boron nitride-loaded iron nanomaterials and sodium persulfate are used to remove different concentrations of oxytetracycline hydrochloride. The specific process and results are as follows:
[0047] Configure a series of oxytetracycline hydrochloride solutions with concentrations of 0.04, 0.08, 0.16 and 0.20mmol / L respectively, take 50mL of them in the reaction device, and then add 100mg / L of porous boron nitride loaded iron material and 150mg / L of Sodium sulfate reacted for 5 minutes, and samples were taken at 0, 1, 2, 3, and 5 minutes. The result is as Figure 4 As shown, the oxytetracycline hydrochloride solution of 0.04-0.20 mmol / L can be effectively catalyzed and oxidized by the porous boron nitride-supported iron material, and the oxidation efficiencies are 92.6%, 90.1%, 88.4% and 83.6%, respectively.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| oxidation efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com