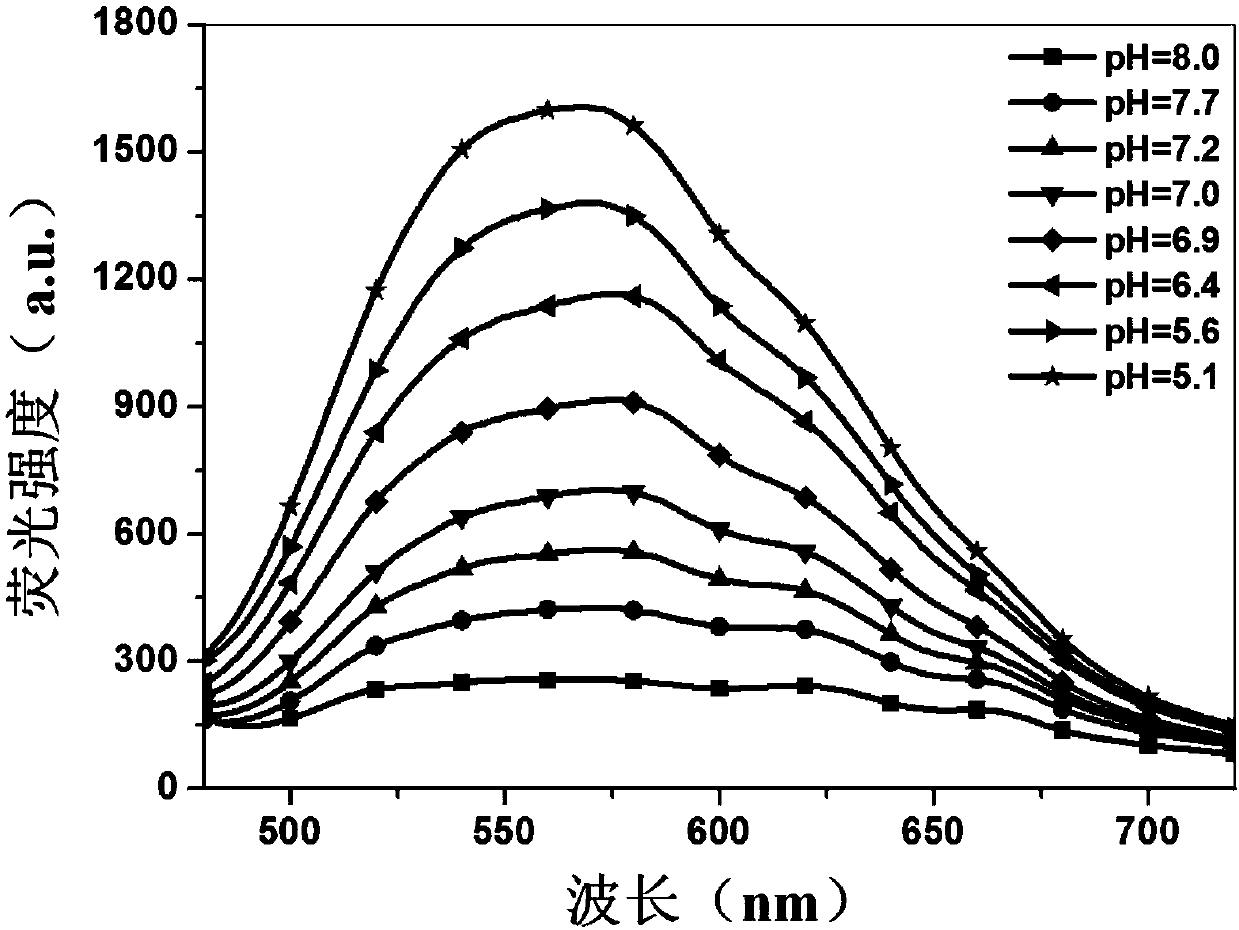
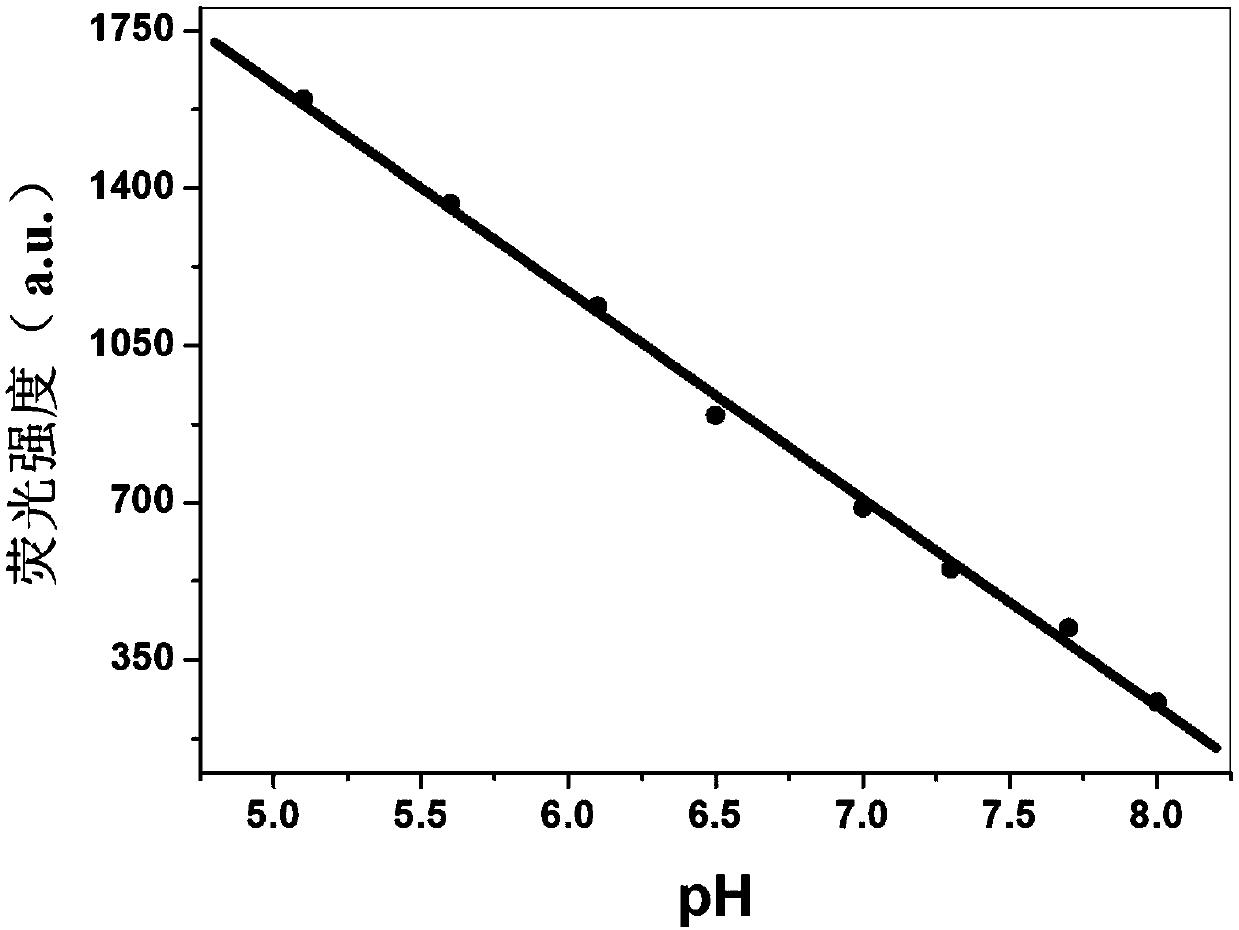
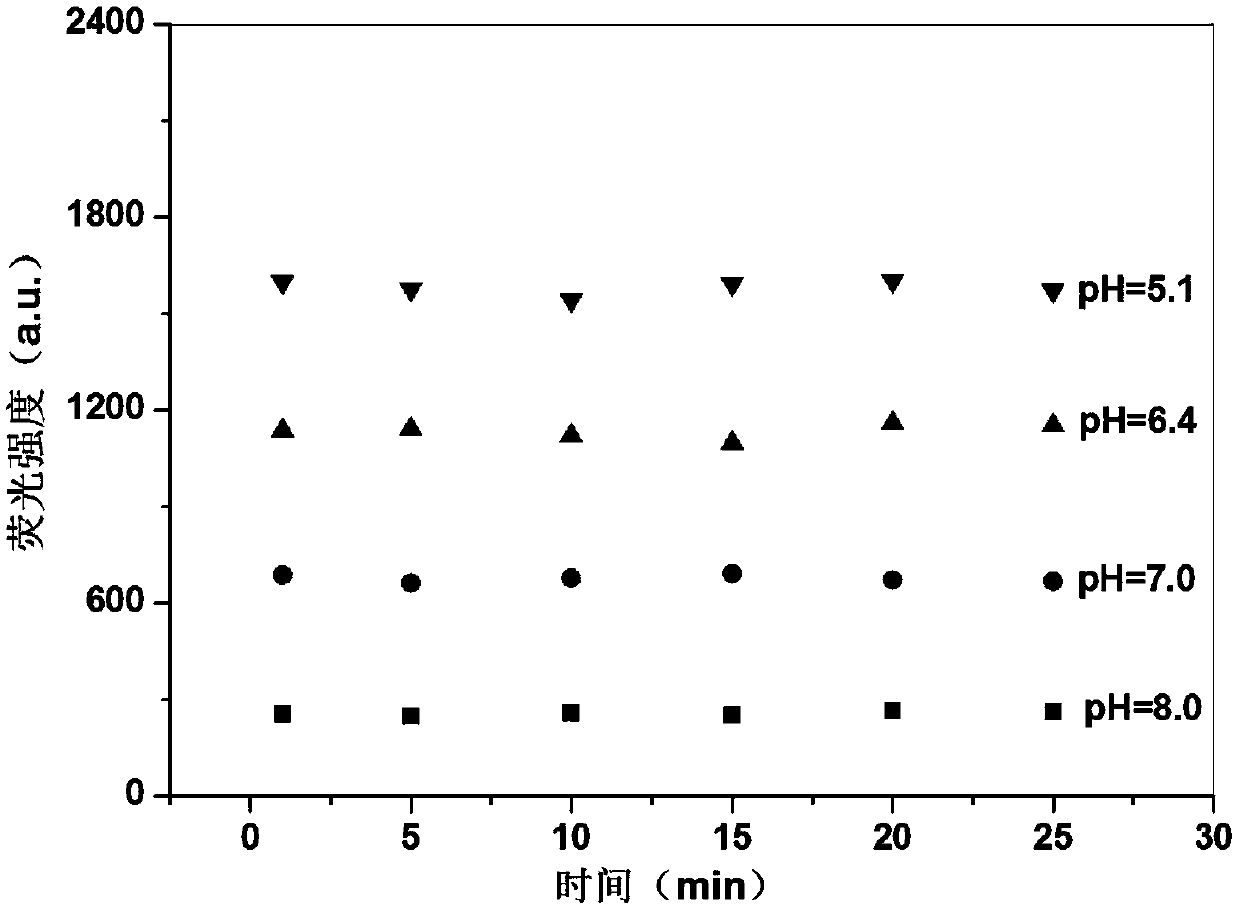
PH-responsive conjugated polymer nanoparticles, preparation method and applications thereof
A technology of polythiophene derivatives and products, applied in the field of fluorescence sensing and detection, can solve the problems of small displacement of stocks, inability to track and observe, poor photostability of probes, etc., and achieve the effects of high accuracy, low toxicity and mild conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] The preparation method of poly 3-(3-N, N'-diethyl) propoxy-4-methylthiophene, comprises the following steps:
[0057] Step 1, add 3-bromo-4-methylthiophene (2.5g, 14mmol) into 3.8mL NMP (N-methylpyrrolidone), and after it is completely dissolved, solution A is obtained, and solution A is added to 20mL containing methanol Sodium in methanol solution to obtain solution B, add cuprous bromide (1.5g, 10mmol) to solution B, reflux reaction under nitrogen protection for 24h, obtain solution C; cool solution C to room temperature 20-25°C, filter , spin the filtrate to dryness, then add 30 mL of dichloromethane to dissolve, wash with water 3 times to obtain the first organic phase liquid, add anhydrous magnesium sulfate to the first organic phase liquid to dry the first organic phase liquid, filter, The first organic phase liquid is spin-dried to obtain product D as a crude product, wherein the structural formula of 3-bromo-4-methylthiophene is:
[0058] In step 1, the produ...
Embodiment 2
[0065] The preparation method of poly 3-(4-N, N'-diethyl) butylthio-4-methylthiophene, comprises the following steps:
[0066] Step 1, add 3-bromo-4-methylthiophene (2.5g, 14mmol) into 3.8mL NMP (N-methylpyrrolidone), and after being completely dissolved, solution A is obtained, and solution A is added to 20mL containing formazan In the methanol solution of sodium thiolate, solution B was obtained, and cuprous bromide (1.5g, 10mmol) and butyllithium (60mg, 9.3mmol) were added to solution B, and reflux reaction was carried out under nitrogen protection for 24h to obtain solution C; Cool solution C to room temperature 20-25°C, filter, spin the filtrate to dryness, add 30 mL of dichloromethane to dissolve, wash with water 3 times to obtain the first organic phase liquid, add anhydrous magnesium sulfate to the first organic phase liquid, For drying the first organic phase liquid, filter, and spin dry the first organic phase liquid to obtain product D as a crude product, wherein th...
Embodiment 3
[0074] The preparation method of poly 3-(5-N, N'-dimethyl)pentylthiophene comprises the following steps:
[0075] Step 1, 3-bromothiophene (2.5g, 15.3mmol) was added to 3.8mL NMP (N-methylpyrrolidone), and after being dissolved, solution A was obtained, and solution A was added to 20mL methanol containing sodium methylthiolate In solution, obtain solution B, in solution B, add cuprous bromide (1.5g, 10mmol) and butyllithium (60mg, 9.3mmol), reflux reaction 24h under nitrogen protection, obtain solution C; Solution C is cooled to Room temperature 20-25°C, filter, spin the filtrate to dry, then add 30mL dichloromethane to dissolve, wash 3 times with water to obtain the first organic phase liquid, add anhydrous magnesium sulfate to the first organic phase liquid to dry the second organic phase liquid An organic phase liquid, filtered, and the first organic phase liquid was spin-dried to obtain product D;
[0076] After step 1, the product D as a crude product was purified by col...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com