Injectable glucose response self-healing hydrogel, and preparation method and application thereof
A glucose-responsive, hydrogel technology, applied in pharmaceutical formulations, medical formulations with non-active ingredients, and medical formulations containing active ingredients, etc. problem, to achieve the effect of good biocompatibility, reduced risk of leakage, and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
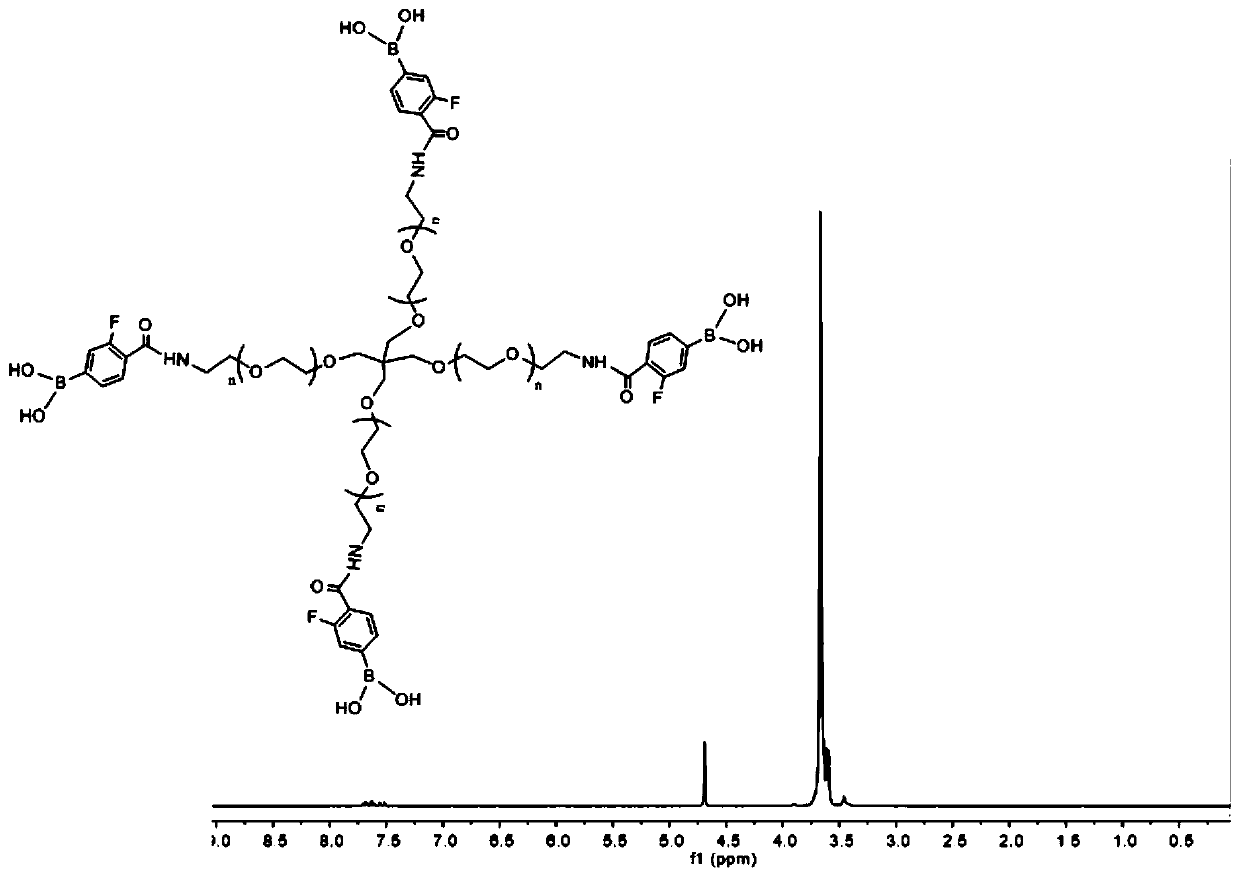
[0056] Preparation of phenylboronic acid-based modified polymer (FPBA-PEG) and polyvinyl alcohol 0588 (PVA) glucose-responsive hydrogel based on 4-carboxy-3-fluoro-phenylboronic acid modified 4-arm-polyethylene glycol-amino preparation
[0057] (1) Synthesis of FPBA-PEG
[0058] 1 g (0.2 mmol) of 4-arm-polyethylene glycol-amino was added to a 50 ml round bottom flask and dissolved in dichloromethane, followed by 0.441 g (2.4 mmol) of 4-carboxy-3-fluoro-phenylboronic acid (FPBA), 0.367g (2.4mmol) 1-hydroxybenzotriazole monohydrate (HOBt), 0.910g (2.4mmol) benzotriazole-N,N,N',N'-tetramethylurea Hexafluorophosphate (HBTU), 0.67 mL of triethylamine, followed by N,N-dimethylformamide (DMF) was added to dissolve HOBt, HBTU, and FPBA. After reacting at room temperature for 24 hours, dichloromethane was removed by rotary evaporation, and the reactant was transferred to a dialysis bag with a molecular weight cut-off of 2 kDa, and dialyzed in deionized water for 24 hours, changing th...
Embodiment 2
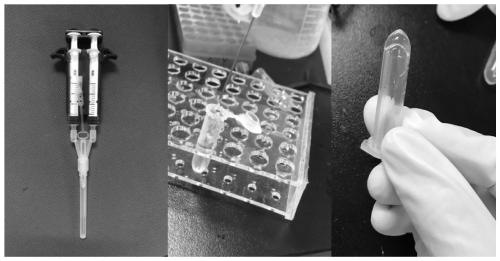
[0062] Preparation of In Situ Formed Hydrogels by Dual Syringes
[0063] Mix the prepared 0.1 ml FPBA-PEG solution (10% w / v, 15% w / v, 20% w / v) with 0.1 ml PVA (10% w / v) solution via a double syringe to form in situ molding gel. figure 2 Photographs for the preparation of in situ formed hydrogels using a dual syringe. Utilize scanning electron microscope to analyze the hydrogel that obtains in embodiment two, obtain its scanning electron micrograph as image 3 shown.
[0064] From image 3 It can be observed that as the solid content of the hydrogel increases, the number of phenylboronic esters formed in the system increases, and the cross-linking density of the gel increases, showing that the grid is tighter, and the grid density of Gel-15 is the smallest. Gel-10 has the highest mesh density, and Gel-12.5 mesh density is in between.
Embodiment 3
[0066] Self-healing properties of FPBA-PEG / PVA hydrogel
[0067] Taking Gel-15 as an example to study the self-healing property of the gel, since the gel is colorless and transparent, in order to feel the self-healing of the hydrogel more intuitively, crystal violet, rhodamine B and FITC were added to the gel -insulin, and then bond the three gels together, and the hydrogel will self-heal into a complete whole within 60s. Photos of hydrogel self-healing such as Figure 4 shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| alcoholysis degree | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com