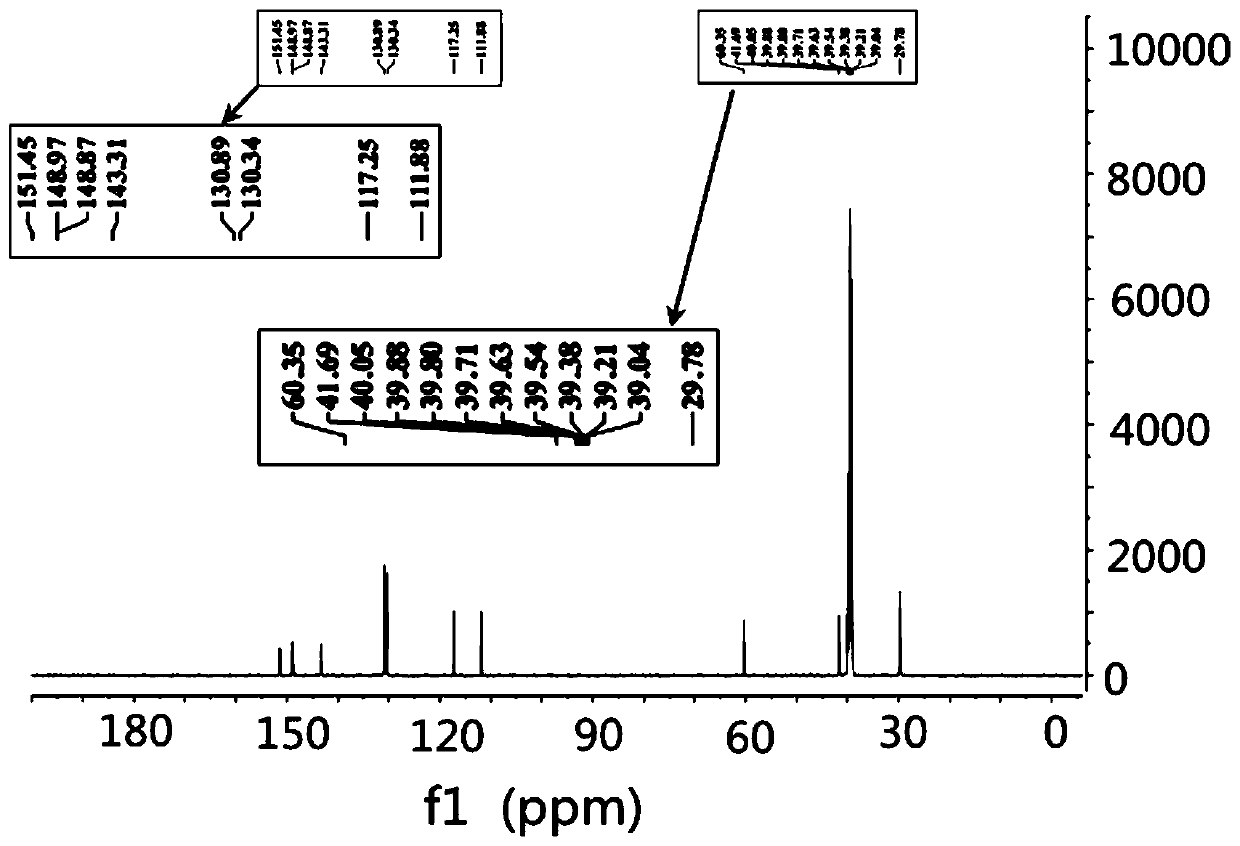
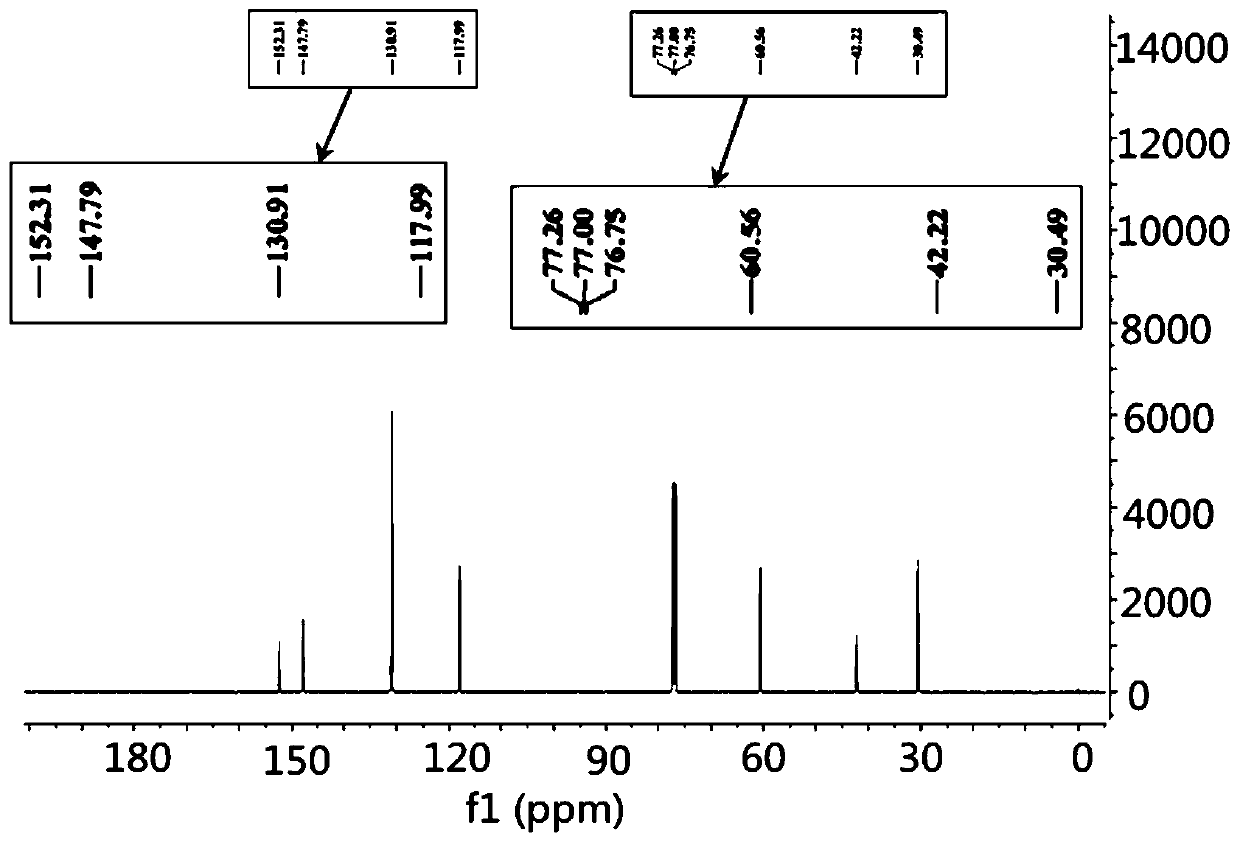
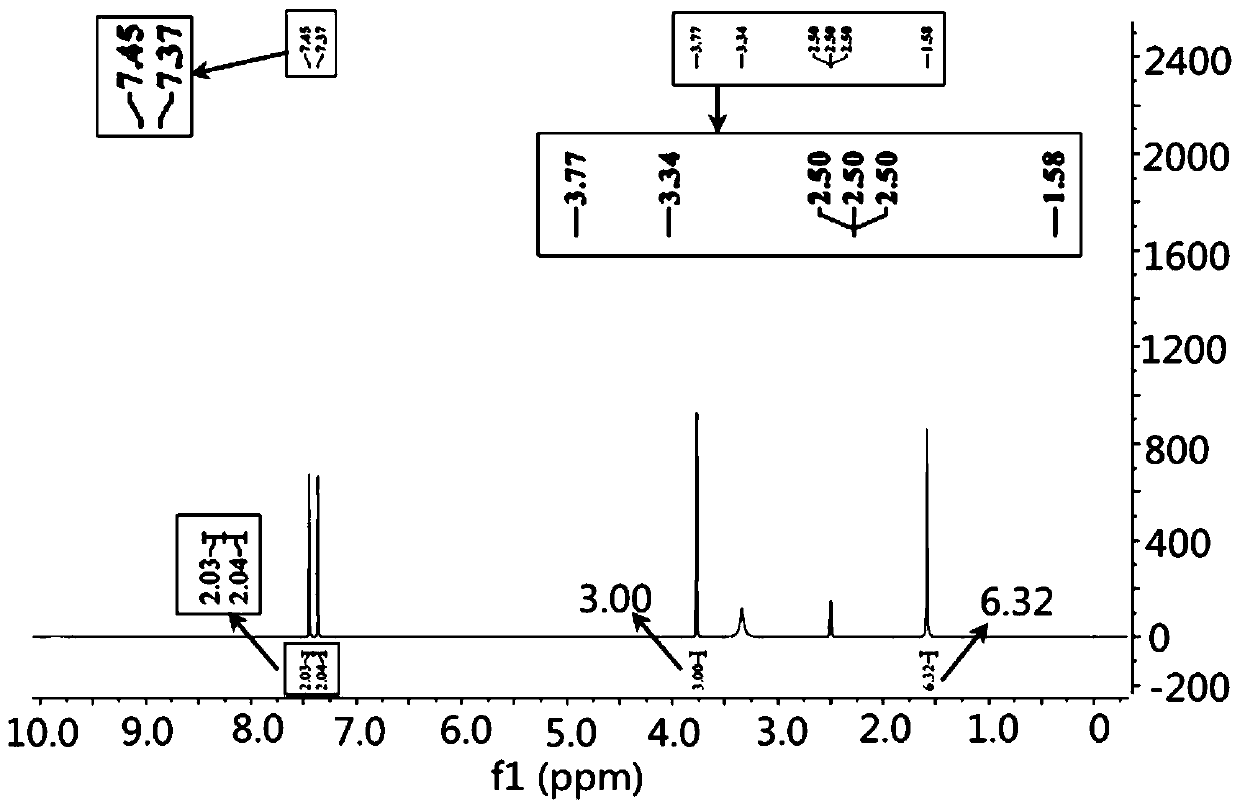
Preparation method for simultaneously synthesizing tetrabromobisphenol A monomethyl ether and dimethyl ether
A technology of tetrabromobisphenol and monomethyl ether, which is applied in the field of preparation of simultaneous synthesis of tetrabromobisphenol A monomethyl ether and dimethyl ether, and can solve the problems of high price, low yield, complicated preparation method and the like , to achieve the effect of low cost, high yield and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] A preparation method for simultaneously synthesizing tetrabromobisphenol A monomethyl ether and dimethyl ether, comprising the steps:
[0031] Take 1g (1.8mmol) of TBBPA in a 25mL pear-shaped flask, add 20mL of acetonitrile to fully dissolve, then add 0.15g (3.6mmol) of sodium hydroxide and vortex to mix, and quickly add 345μL of iodomethane (5.4mmol, ρ=2.28 g / mL), stirred magnetically at room temperature for 15 minutes, heated to 60°C and stirred and refluxed for 4 hours. After the reaction, cooled in an ice bath to terminate the reaction, dissolved and washed with 5 mL of dichloromethane for several times, and filtered to remove sodium hydroxide. After removing water with sodium sulfate, filter and transfer to another pear-shaped bottle, use a rotary evaporator to reduce pressure and rotate to a small volume to obtain a concentrated solution, in which there are unreacted TBBPA, tetrabromobisphenol A monomethyl Tetrabromobisphenol A dimethyl ether and tetrabromobisphen...
Embodiment 2
[0037] A preparation method for simultaneously synthesizing tetrabromobisphenol A monomethyl ether and dimethyl ether, comprising the steps:
[0038] Take 1g (1.8mmol) of TBBPA in a 25mL pear-shaped flask, add 20mL of acetonitrile to fully dissolve, then add 0.15g (3.6mmol) of sodium hydroxide and vortex to mix, and quickly add 115μL of iodomethane (1.8mmol, ρ=2.28 g / mL), magnetically stirred at room temperature for 15 minutes, heated to 60°C and stirred and refluxed for 4 hours, after the reaction was completed, cooled in an ice bath to terminate the reaction, dissolved and washed with 5 mL of dichloromethane several times, filtered to remove sodium hydroxide, and a small amount of anhydrous After removing water by sodium sulfate, filter and transfer to another pear-shaped bottle, use a rotary evaporator to reduce the pressure and rotate to a small volume to obtain a concentrated solution, in which there are unreacted TBBPA, tetrabromobisphenol A monomethyl ether and tetrabro...
Embodiment 3
[0043] A preparation method for simultaneously synthesizing tetrabromobisphenol A monomethyl ether and dimethyl ether, comprising the steps:
[0044] Take 1g (1.8mmol) of TBBPA in a 25mL pear-shaped flask, add 20mL of acetonitrile to fully dissolve, then add 0.15g (3.6mmol) of sodium hydroxide and vortex to mix, and quickly add 345μL of iodomethane (5.4mmol, ρ=2.28 g / mL), magnetically stirred at room temperature for 15 minutes, heated to 60°C and stirred for 2 hours under reflux. After removing water by sodium sulfate, filter and transfer to another pear-shaped bottle, use a rotary evaporator to reduce the pressure and rotate to a small volume to obtain a concentrated solution, in which there are unreacted TBBPA, tetrabromobisphenol A monomethyl ether and tetrabromobisphenol A dimethyl ether; the relative percentages of tetrabromobisphenol A monomethyl ether and tetrabromobisphenol A dimethyl ether in the concentrate are 62.5% and 25% respectively.
[0045] According to the m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com