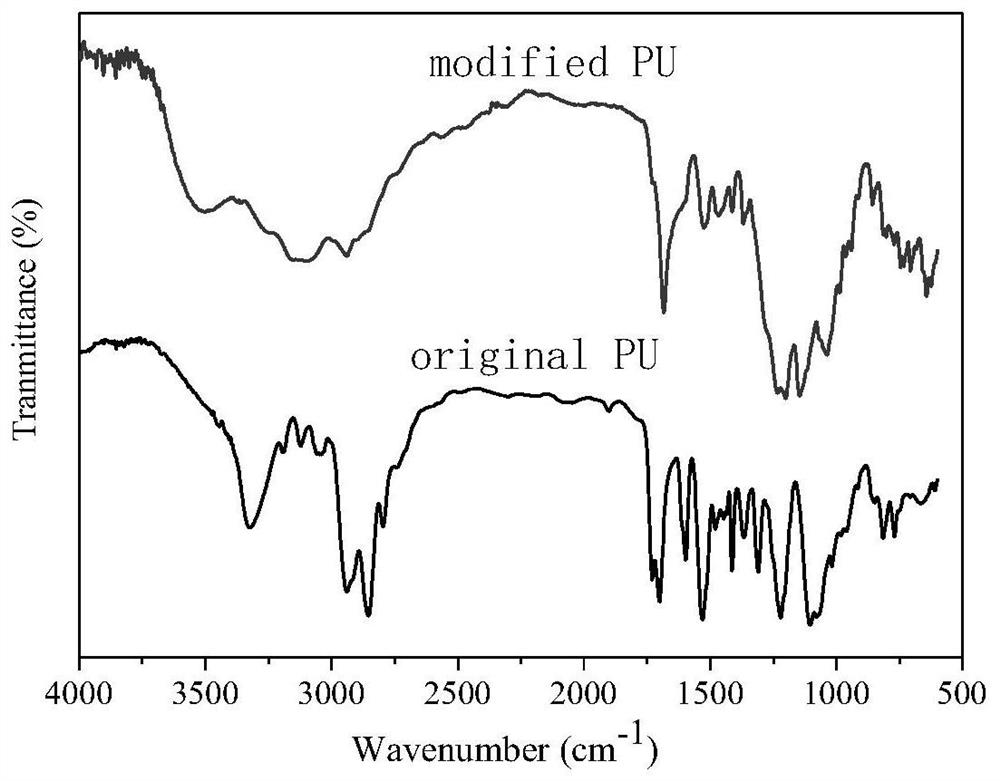
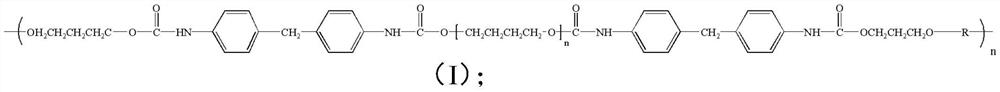A kind of fluorine-containing polyurethane and preparation method thereof
A technology of polyurethane and sulfonyl fluoride, which is applied in the field of fluorine-containing polyurethane and its preparation, and can solve the problems of high unit price of fluorine-containing monomers, difficult synthesis, and restrictions on wide application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The present invention also provides a kind of preparation method of above-mentioned fluorine-containing polyurethane, comprises the following steps:
[0042] A) mixing polyurethane having a structure represented by formula (IV) with a polar solvent to obtain a first mixed solution;
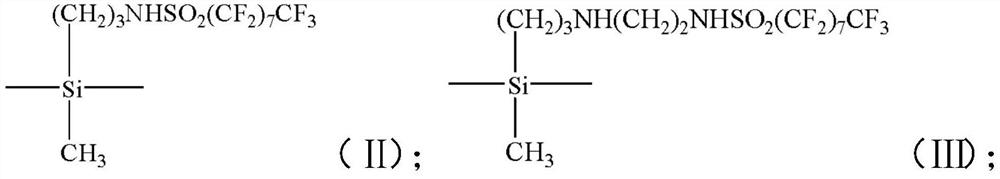
[0043] B) mixing the first mixed solution with the silane coupling agent to obtain the second mixed solution;
[0044] C) mixing the second mixed solution, sulfonyl fluoride and water, and reacting to obtain a fluorine-containing polyurethane having a structure shown in formula (I);
[0045]
[0046] Among them, 10≤n≤20.
[0047] The present invention has no special limitation on the source of the polyurethane having the structure represented by formula (IV). In some embodiments of the present invention, the polyurethane having the structure shown in formula (IV) is prepared according to the following method:
[0048] performing a chain extension reaction on the polyurethane prepolyme...
Embodiment 1
[0102] Have the preparation of the polyurethane of structure shown in formula (IV):
[0103] (1) Dehydration treatment of diisocyanate
[0104] Add 2.55g (0.01mol) MDI into a three-necked flask equipped with mechanical stirring, nitrogen protection, and a constant pressure dropping funnel, heat in an oil bath and vacuumize with a vacuum pump until the dehydration is complete.
[0105] (2) Prepolymerization reaction
[0106] Nitrogen was passed into the three-necked flask in advance, and then the temperature in the three-necked flask was controlled to be 80°C. 5g of PTMG (0.005mol) was added dropwise to the three-necked flask with a constant pressure dropping funnel at a rate of 3 drops / s. After completion, add 9 mL of toluene, the solid content is 49.20%, and react for 3 hours to obtain the polyurethane prepolymer.
[0107] (3) Chain extension reaction
[0108] After the prepolymerization, the NCO% titration was carried out by the di-n-butylamine method, and then the BDO ch...
Embodiment 2
[0117] The preparation method of the polyurethane having the structure shown in formula (IV) is the same as in Example 1.
[0118] Preparation of fluorine-containing polyurethane:
[0119] Weigh 1g of polyurethane (n=13) having the structure shown in formula (IV), place it in a 100mL Erlenmeyer flask, then pour 15mL of N,N-dimethylformamide (DMF), and control the stirring speed to 230r / min, stirred at room temperature for 0.5h.
[0120] Then weigh 0.26g of KH902 and drop it into the conical flask using a constant pressure dropping funnel, control the dropping rate to 1 drop / s, keep the stirring speed constant, and continue stirring at room temperature for 0.4h after the dropping is completed.
[0121] Place the Erlenmeyer flask in a cold water bath, cool to 5°C, weigh 0.45g of perfluorooctanesulfonyl fluoride, drop it into the Erlenmeyer flask using a constant pressure dropping funnel, and control the dropping rate to 2 drops / min. Then 0.03 g of distilled water was added dr...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Hydrophobic angle | aaaaa | aaaaa |
| Modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com