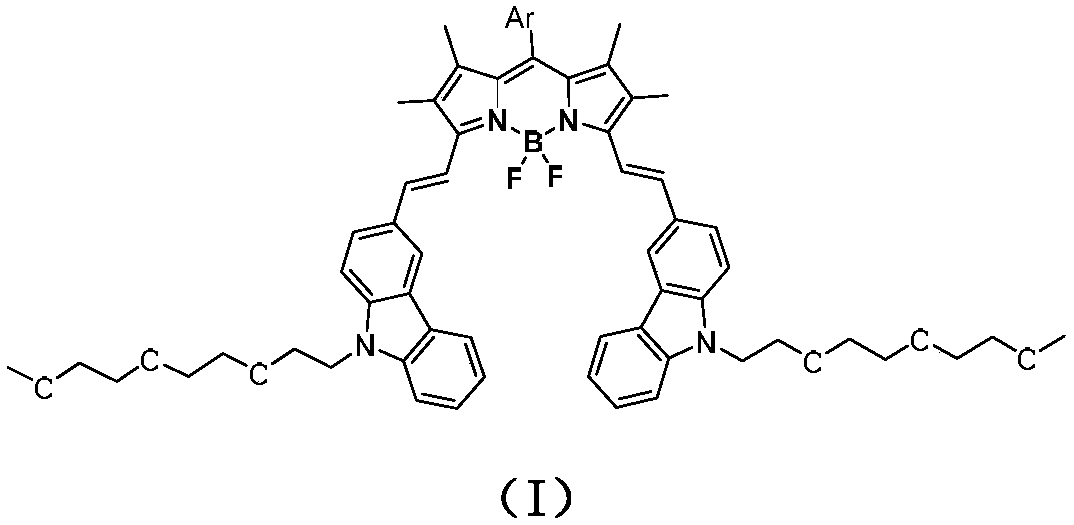
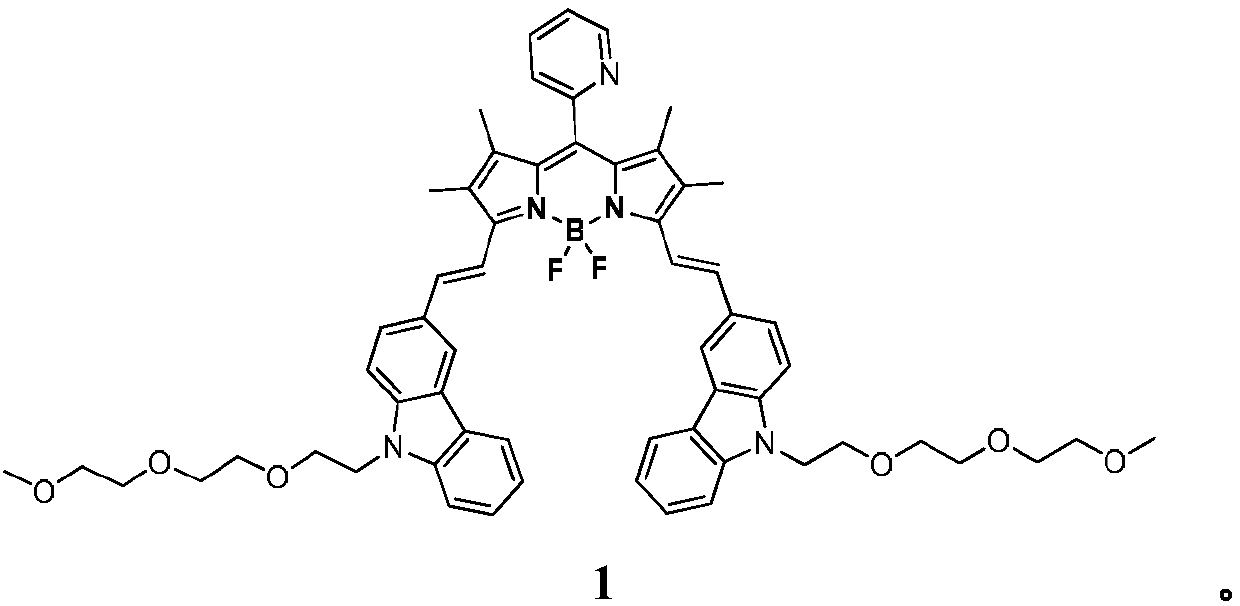
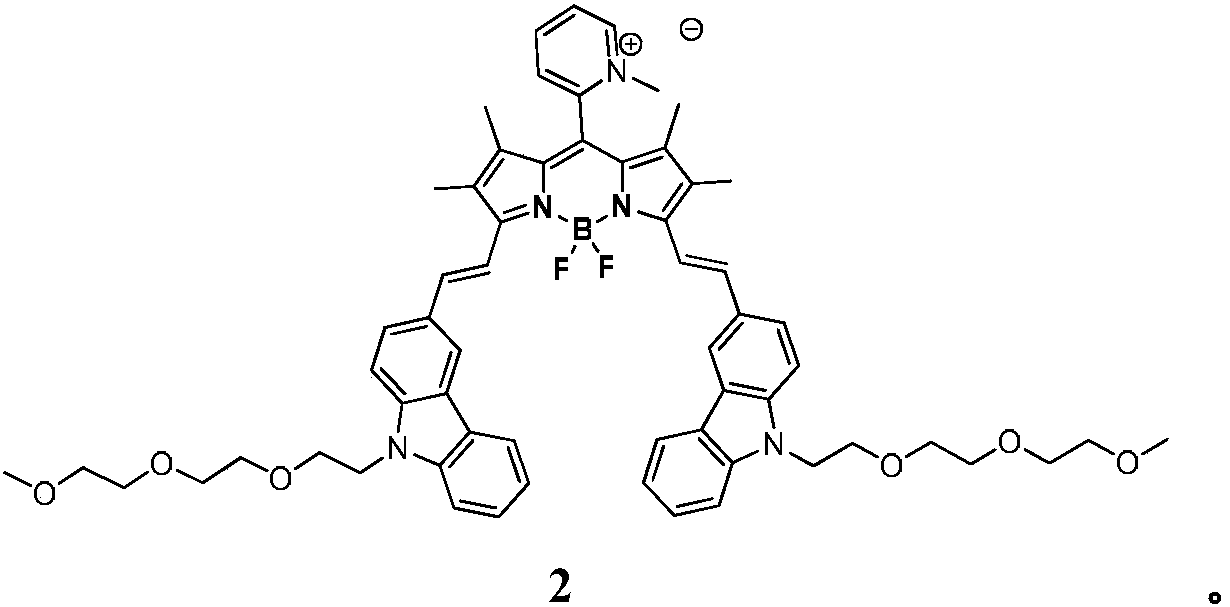
Pyridine-containing BODIPY and quaternary ammonium salt photosensitizer thereof, and preparation method and applications of quaternary ammonium salt photosensitizer
A technology of fluoroborate dipyrrole and quaternary ammonium salts, which is applied in the field of drug synthesis, and can solve the problems of easy mutation of tumor cell growth, multidrug resistance of chemotherapy, and failure of chemotherapy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1 synthetic compound 1
[0023] 1) Synthesis of 2-(2-(2-methoxyethoxy)ethoxy)ethyl 4-methylbenzenesulfonate
[0024] 2g 2-(2-(2-methoxyethoxy)ethoxy)ethanol and 2.2g p-toluenesulfonic acid are dissolved in the mixed solution of 80mL dichloromethane and 80mL tetrahydrofuran, drop into 1.4mL triethylamine, argon Protected under gas, stirred at room temperature for 24 h, TLC monitored the completion of the reaction, evaporated THF and DCM under reduced pressure, added 150 ml each of ethyl acetate and water to shake the layers, extracted the aqueous layer with ethyl acetate, combined the organic layers, washed with saturated sodium chloride, Dry over anhydrous sodium sulfate, distill off the solvent under reduced pressure, and purify by column chromatography, PE:EA=1:4 elution gives the purified product 3.16g, yield 81.6%, MS (ESI) m / z ([M+H ] + ):319.1;
[0025] 2) Synthesis of 9-(2-(2-(2-methoxyethoxy)ethoxy)ethyl)-9H-carbazole
[0026] Dissolve 2.4 g of ca...
Embodiment 2
[0036] Embodiment 2 synthetic compound 2
[0037] Put 40 mg of compound 1 in a sealed tube, add 0.5 mL of iodomethane, and react at 60°C for 12 hours. After the iodomethane is completely volatilized, add a small amount of DCM to make it just dissolve, add methyl tert-butyl ether to precipitate a purple-black solid, suction filter, and dry , to obtain product 29mg, yield 65%, 1 H NMR (400MHz, CDCl 3 )δ8.68(s,1H),8.59(d,J=16.1Hz,2H),8.42(s,1H),8.35-8.34(m,2H),8.16-8.14(m,2H),8.05(s ,1H),7.89-7.87(m,2H),7.80-7.75(m,2H),7.58-7.56(m,2H),7.50-7.49(m,5H),7.26-7.25(m,2H),4.54 (s,7H),3.92-3.91(m,4H),3.54-3.53(m,4H),3.51-3.50(m,4H),3.46-3.65(m,4H),3.42-3.40(m,4H) ,3.32(s,6H),1.36(s,6H).
Embodiment 3
[0038] Embodiment 3: synthetic compound 3
[0039] 1) Synthesis of 1,3,5,7-tetramethyl-8-(3-pyridyl)-4,4'-difluoroborondipyrrole
[0040] 470 mg of 3-pyridinecarbaldehyde and 920 mg of 2,4-dimethylpyrrole were dissolved in 250 mL of dry DCM, a catalytic amount of TFA was added, protected by argon, and stirred at room temperature for 24 hours. Part of the DCM was distilled off under reduced pressure until the volume of the solution was 70 mL, 1.5 g of 2,3-dichloro-5,6-dicyano-p-benzoquinone was added, protected by argon, and stirred at room temperature for 2 hours. Add 7 mL of triethylamine and 7 mL of boron trifluoride ether solution, under argon protection, stir overnight at room temperature, remove the solvent under reduced pressure, dissolve the residual solid in 150 mL of DCM, wash with water three times, dry over anhydrous sodium sulfate, evaporate under reduced pressure Remove the solvent, and purify DCM / PE=1 / 3 by column chromatography to obtain 195 mg of orange solid, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com