Porcine-derived osteopontin (OPN) monoclonal antibody, hybridoma cell strain thereof, and applications of monoclonal antibody and hybridoma cell strain
A technology of hybridoma cell line and monoclonal antibody, which is applied in the field of bioengineering, can solve the problems of not single identified parts, false positives, deep background, etc., and achieve the effect of high application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1: In vitro acquisition of porcine OPN protein
[0034] Referring to Examples 1 and 2 in Chinese patent application 201811476996.8 "A Recombinant Lentiviral Vector, Recombinant Lentivirus and Its Application", the porcine OPN recombinant protein was obtained. Specifically, it includes: predicting the signal peptide of the OPN protein through the signal peptide prediction software SignalP4.1Server, removing its own signal peptide, and adding a 6'His sequence in front of the gene sequence, that is, the final sequence is a 6'His-OPN sequence. Ligate the target sequence into the lentiviral modified vector pCDH-CMV-HSA-MCS-6His-EF1-GFP+Puro to obtain the recombinant lentiviral vector pCDH-CMV-HSA-6′His-OPN-6His-EF1-GFP+Puro . Through the lentiviral packaging system, the packaging plasmids (pMD2.G and psPAX2) and the lentiviral recombinant vector were co-transfected into 293FT cells to obtain the virus containing the target gene. The virus was infected into the CHO-...
Embodiment 2
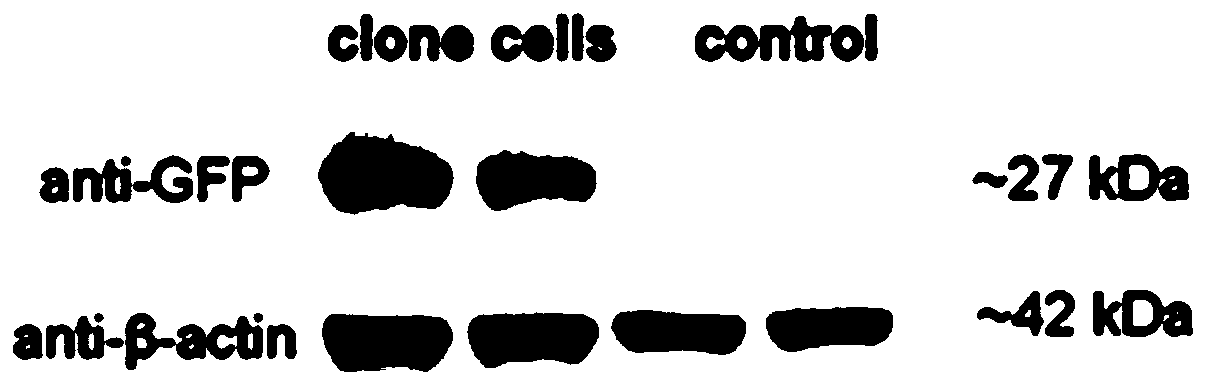
[0035] Example 2: Preparation of porcine OPN mouse monoclonal antibody
[0036] The purified porcine OPN recombinant protein expressed in the eukaryotic system in Example 1 was used as an immunogen to immunize mice. Select purebred BABL / c female mice of SPF grade for 6-8 weeks to immunize with porcine OPN recombinant protein. For the first immunization, 100 μg of porcine OPN recombinant protein plus Freund’s complete adjuvant (mixed in equal volumes) was subcutaneously injected into multiple points; at an interval of three weeks, the second immunization of 100 μg of porcine OPN recombinant protein was subcutaneously injected into multiple points with Freund’s incomplete adjuvant. The total volume is 200 μL; at an interval of three weeks, the third immunization dose is halved, that is, 50 μg of protein without adjuvant is injected intraperitoneally, blood is collected from the orbit 7 days later, and the titer is measured by ELISA. After the titer reaches a certain level, the b...
Embodiment 3
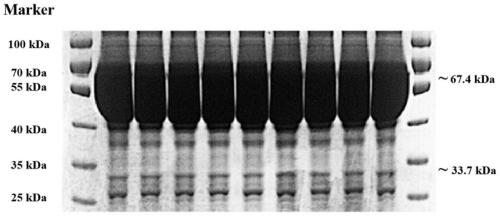
[0067] Example 3 Monoclonal Antibody Purification
[0068] 1. Caprylic acid-ammonium sulfate precipitation crude extraction
[0069] Add 2 parts of 0.06mol / L pH5.0 acetate buffer to 1 part of centrifuged pretreated ascites, adjust the pH to 4.8 with 1mol / L HCl solution; Add octanoic acid, finish adding within 30min, let stand at 4°C for 2h, centrifuge at 2000rpm for 30min, discard the precipitate; filter the supernatant through a nylon sieve (125μm), add 1 / 10 volume of 0.01mol / L PBS buffer, and use 1mol / L Adjust the pH to 7.2 with NaOH; add saturated ammonium sulfate to 45% saturation at 4°C for 30 minutes, let stand for 1 hour; centrifuge at 12000rpm for 30 minutes, discard the supernatant; dissolve the precipitate in an appropriate amount of PBS buffer (containing 137mmol / L NaCl, 2.6mol / L KCl, 0.2mmol / L EDTA (ethylenediaminetetraacetic acid)), dialyze in 50-100 times volume of PBS buffer, overnight at 4°C, change water more than 3 times during the period; centrifuge at 120...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com