Preparation method for D-type rare ketohexose
A rare ketohexose, D-type technology, applied in the field of preparation of D-type rare ketohexose, can solve the problems of low conversion rate, difficult separation and purification of products, etc., and achieve the effect of low production cost, low price and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
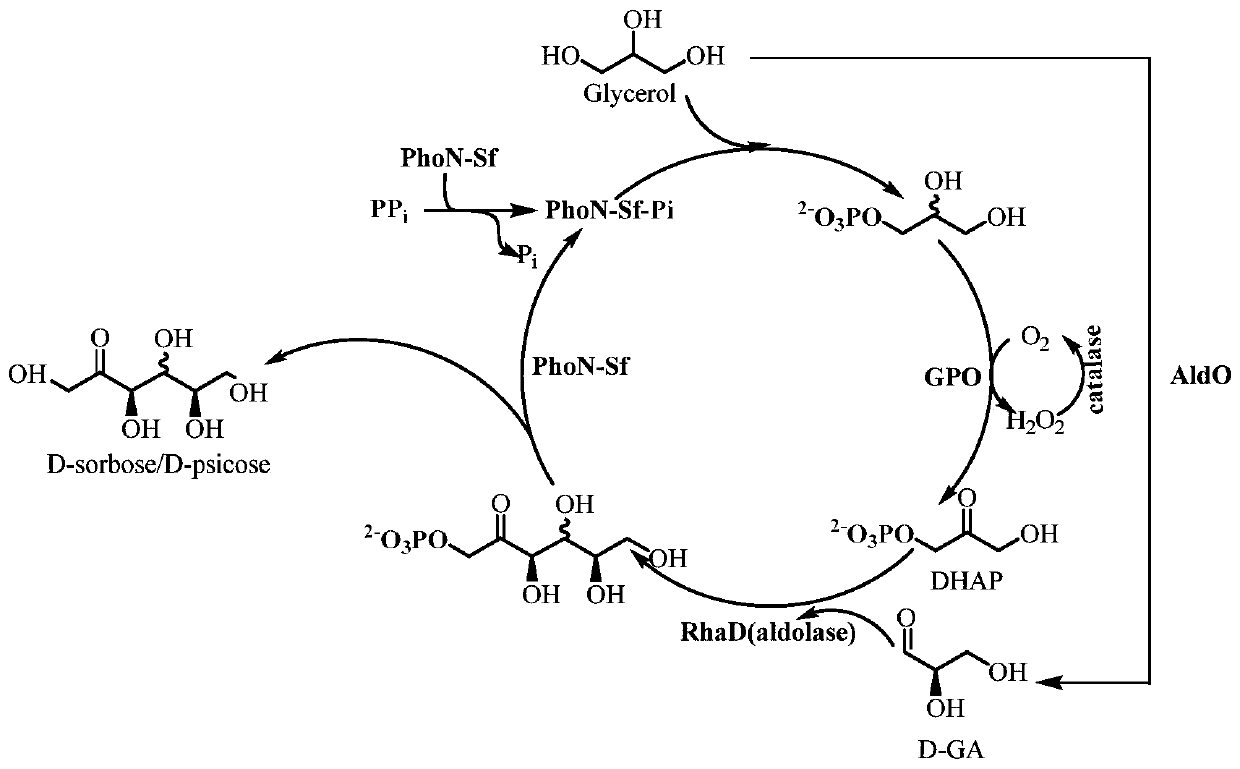
[0034] The preparation method of D-psicose and D-sorbose of the present invention comprises the following steps: (1) using glycerol and pyrophosphate as substrates, adding L-rhamnosugar-1-phosphate aldolase (L-rhamnosin) -rhamnulose-1-phosphate aldolase, EC4.1.2.19), glycerol phosphate oxidase (L-α-glycerophosphate oxidase, EC: 1.1.3.21) catalase (Catalase, EC: 1.11.1.6), acid phosphatase (acid phosphatase, EC: 3.1.3.2) multi-enzyme catalyst of sugar alcohol oxidase (alditol oxidase, EC: 1.1.3.41) Establish a multi-enzyme reaction system for enzymatic reactions. (2) Separating and purifying the product of the enzyme-catalyzed reaction to obtain the product.
[0035] Wherein, the glycerol concentration in step (1) is 300-800mM; preferably, the glycerol concentration is 500-800mM, most preferably 700mM; the pyrophosphate concentration is 20-100mM; most preferably 40mM; wherein The pyrophosphoric acid is pyrophosphate, preferably disodium dihydrogen pyrophosphate: tetrasodium py...
experiment example 1
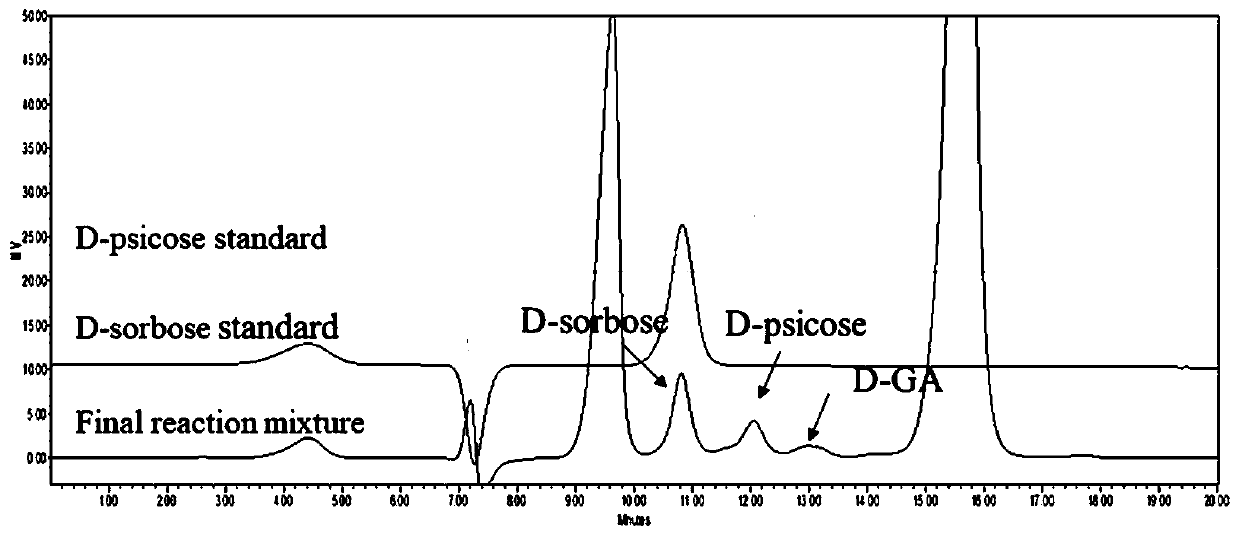
[0045] Experimental example 1: In vitro multi-enzyme catalyzed conversion of glycerol to D-psicose and D-sorbose
[0046] Glycerol was converted into D-psicose and D-sorbose ( figure 1 ). These key enzymes include: (1) acid phosphatase (PhoN-Sf, EC: 3.1.3.2), which phosphorylates glycerol to generate DL-3-phosphate glycerol. At the same time, sugar phosphate is dephosphorylated to generate rare ketose; (2) glycerol phosphate oxidase (GPO, EC: 1.1.3.21), which catalyzes DL-3-glycerol phosphate to dihydroxyacetone phosphate (DHAP); (3) sugar alcohol Oxidase (AldO, EC: 1.1.3.41), converts glycerol to D-glyceraldehyde; (4) L-rhamnosan-1-phosphate aldolase (RhaD, EC 4.1.2.19), converts DHAP and D-glyceraldehyde is sugar phosphate; (5) catalase (Catalase, EC: 1.11.1.6), which decomposes hydrogen peroxide into water and oxygen.
[0047] In the present invention, acid phosphatase is derived from Shigella flexneri (Shigelaflexneri), and its gene sequence is numbered CP0190 on KEGG, ...
experiment example 2
[0061] Experimental Example 2: Using L-fucose-1-phosphate aldolase to convert glycerol into D-psicose and D-sorbose in vitro with multi-enzyme catalysis
[0062] L-fuculose-1-phosphate aldolase (L-fuculose-1-phosphate aldolase, EC 4.1.2.17) and L-rhamnosin-1 derived from Thermus thermophilus HB8 -Phosphate aldolase (RhaD, EC4.1.2.19) has the same catalytic activity, so the present invention adds L-fucose-1-phosphate aldolase to the multi-enzyme catalytic system.
[0063] In the present invention, glycerol is converted into D-psicose and D-sorbose ( Figure 5 ). L-fucose-1-phosphate aldolase from Thermus thermophilus (Thermus thermophilus) HB8, its gene sequence number on NCBI is 2827875, codon optimization was carried out according to the codon preference of Escherichia coli ; The corresponding expression vector pET28a-fucA was obtained by molecular cloning. This plasmid was transformed into Escherichia coli expression bacteria BL21 (DE3), and protein expression and purific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com