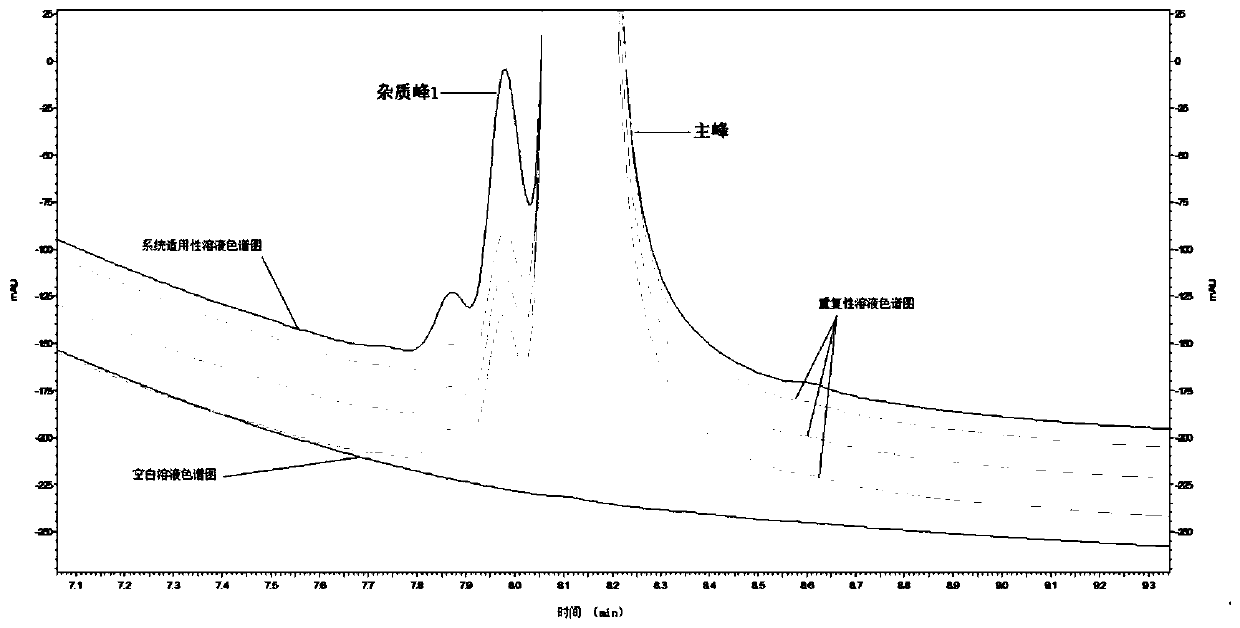
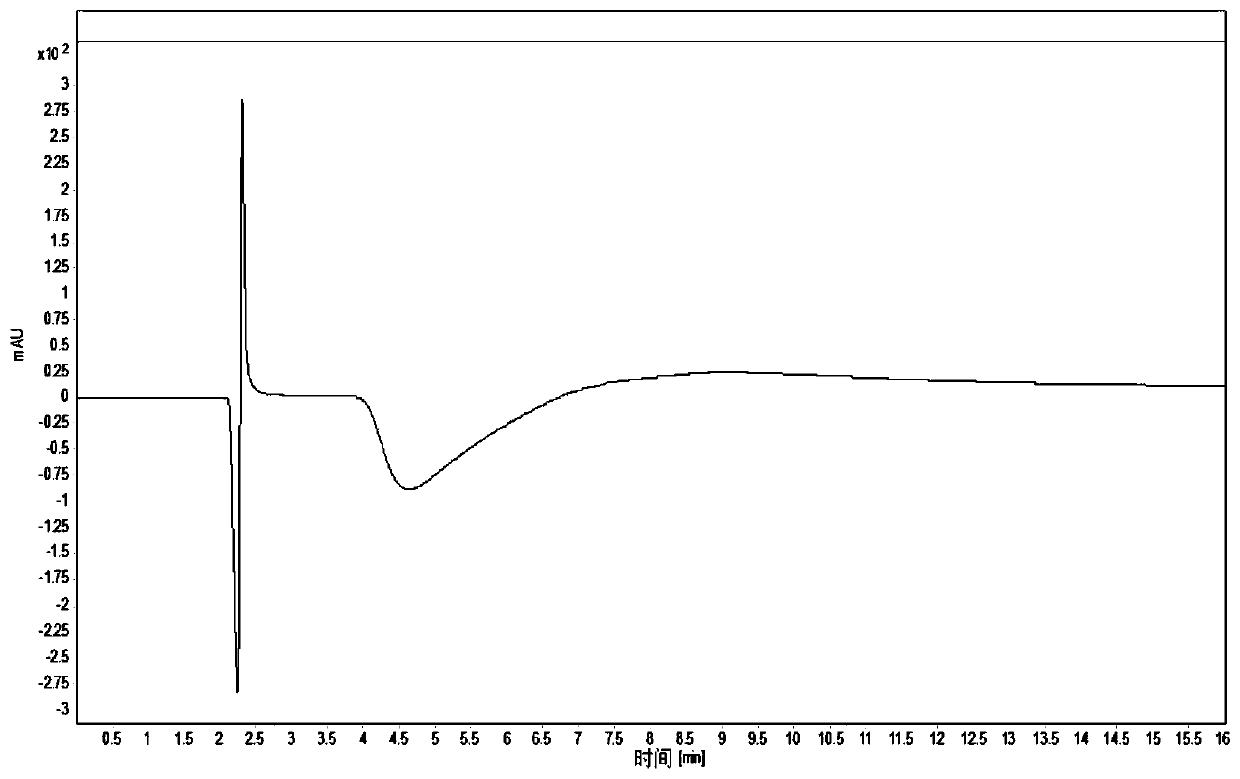
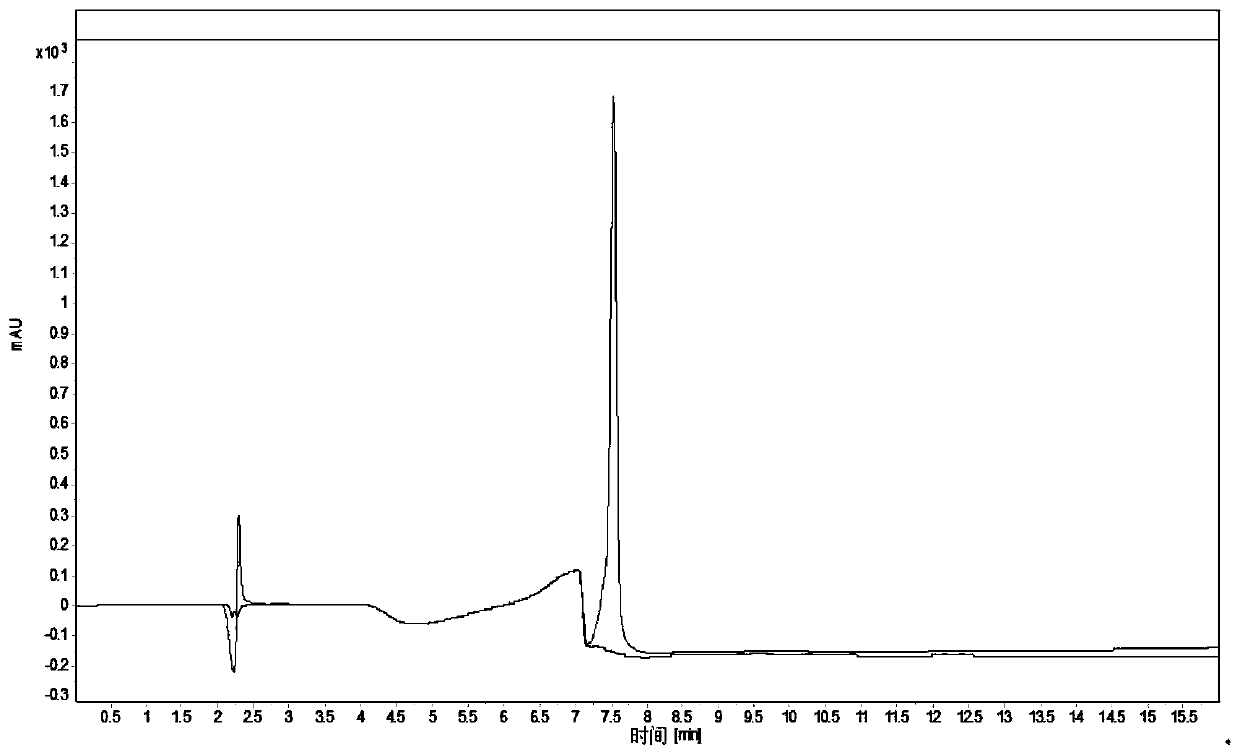
High performance liquid chromatography detection method of recombinant protein drug charge heterogeneous impurities
A technology of high performance liquid chromatography and detection method, which is applied in the field of high performance liquid chromatography detection of impurities with heterogeneity in charge of recombinant protein drugs, can solve the problems of deformation, low resolution, complicated operation and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] This embodiment is a detection experiment of charge heterogeneity impurities in insulin glargine injection, and the experimental process is as follows:
[0051] (1) Treatment of the test product: Take 400 μl of insulin glargine injection, add it to 600 μl of 0.01M HCl, mix well, and obtain the sample to be tested;
[0052] (2) Determine the chromatographic conditions:
[0053]Instrument: Agilent 1260 liquid chromatograph equipped with diode array detector;
[0054] Chromatographic column: ProPac WCX-10, BioLC, 4×250mm (Thermo);
[0055] Mobile phase A: 20mM sodium acetate (pH5.6);
[0056] Mobile phase B: 10mM sodium acetate, 0.5M NaCl, 50% (volume percentage) ethanol;
[0057] Flow rate: 0.5ml / min;
[0058] Injection volume: 5μl;
[0059] Column temperature: 30°C;
[0060] Detection wavelength: 214nm;
[0061] Last run: 12min.
[0062] Gradient elution was carried out with mobile phase A and mobile phase B, and the elution procedure was the same as in Table 1. ...
Embodiment 2
[0067] This example is an experiment for detecting charge heterogeneity impurities in insulin degludec injection, and the experiment process is as follows:
[0068] (1) Treatment of the test product: Take 300 μl of insulin degludec injection, add 400 μl of ultrapure water, mix well, and obtain the sample to be tested;
[0069] (2) Determine the chromatographic conditions:
[0070] Instrument: Agilent 1260 liquid chromatograph equipped with diode array detector;
[0071] Chromatographic column: ProPac WCX-10, BioLC, 4×250mm (Thermo);
[0072] Mobile phase A: 20mM sodium acetate (pH5.0);
[0073] Mobile phase B: 10mM sodium acetate, 0.5M NaCl, 50% (volume percentage) ethanol;
[0074] Flow rate: 0.5ml / min;
[0075] Injection volume: 10μl;
[0076] Column temperature: 30°C;
[0077] Detection wavelength: 214nm;
[0078] Last run: 12min.
[0079] Gradient elution was carried out with mobile phase A and mobile phase B, and the elution procedure was the same as in Table 2. ...
Embodiment 3
[0082] In this embodiment, the influence of not adding an organic solvent on the detection effect in the mobile phase B is investigated. The specific experimental process is as follows:
[0083] (1) System suitability solution: Weigh 15.2mg of insulin glargine into a 10ml volumetric flask, add 2ml of 1M HCl, react at room temperature for 4h, add 2ml of 1M NaOH to neutralize, dilute with 0.01M HCl to volume, and mix well to obtain Sample to be tested;
[0084] (2) Determine the chromatographic conditions:
[0085] Instrument: Agilent 1260 liquid chromatograph equipped with diode array detector;
[0086] Chromatographic column: ProPac WCX-10, BioLC, 4×250mm (Thermo);
[0087] Mobile phase A: 20mM sodium acetate (pH5.6);
[0088] Mobile phase B: 10mM sodium acetate, 0.5M NaCl;
[0089] Flow rate: 0.5ml / min;
[0090] Injection volume: 5μl;
[0091] Column temperature: 30°C;
[0092] Detection wavelength: 214nm;
[0093] Last run: 12min.
[0094] Gradient elution was carri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| percent by volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com