Triamine derivative and organic electroluminescent device thereof
An amine derivative and electroluminescence technology, which is applied in the fields of electric solid devices, electrical components, organic chemistry, etc., can solve the problems of blocking electrons and low luminous efficiency of organic electroluminescence devices, and achieve the effect of improving luminous efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0102] Compound preparation
[0103] Description of raw materials and reagents: The raw materials and reagents used in the present invention are reagent pure.
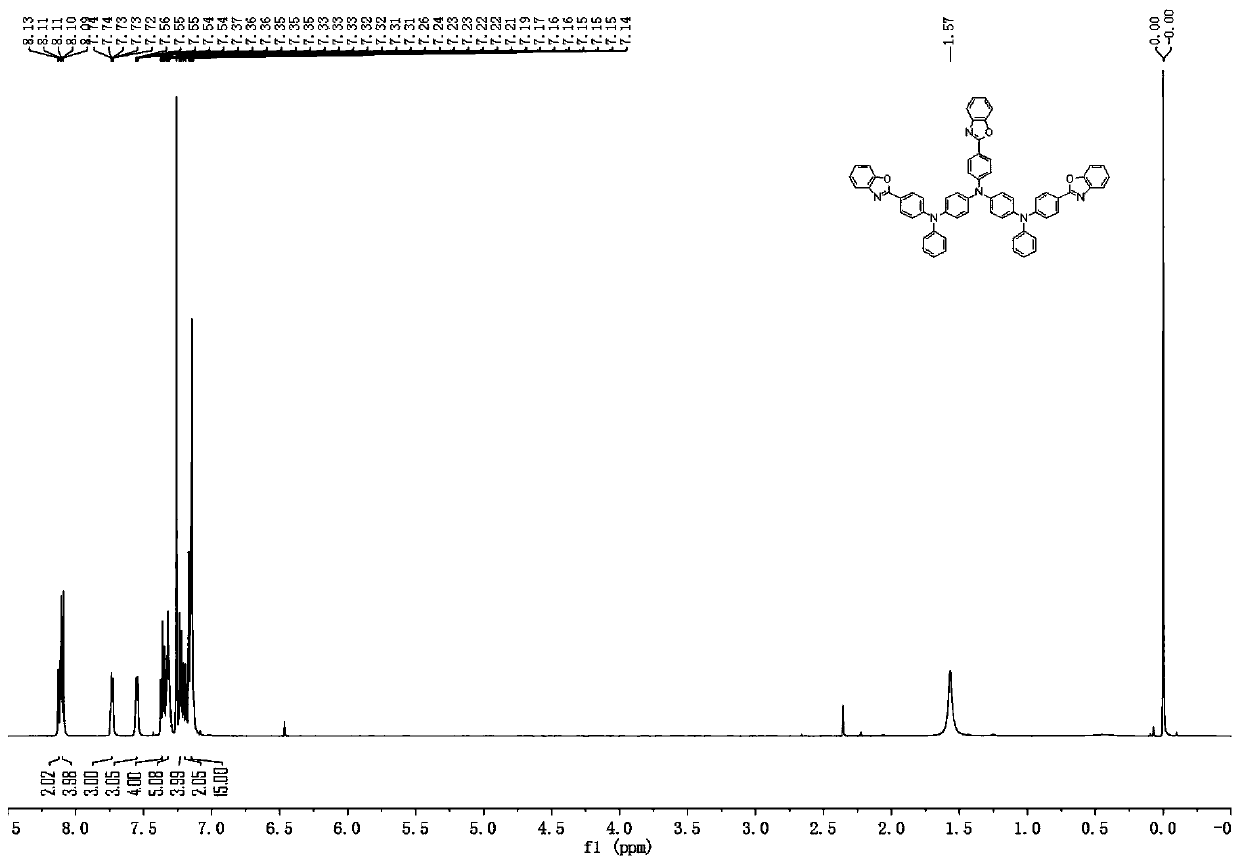
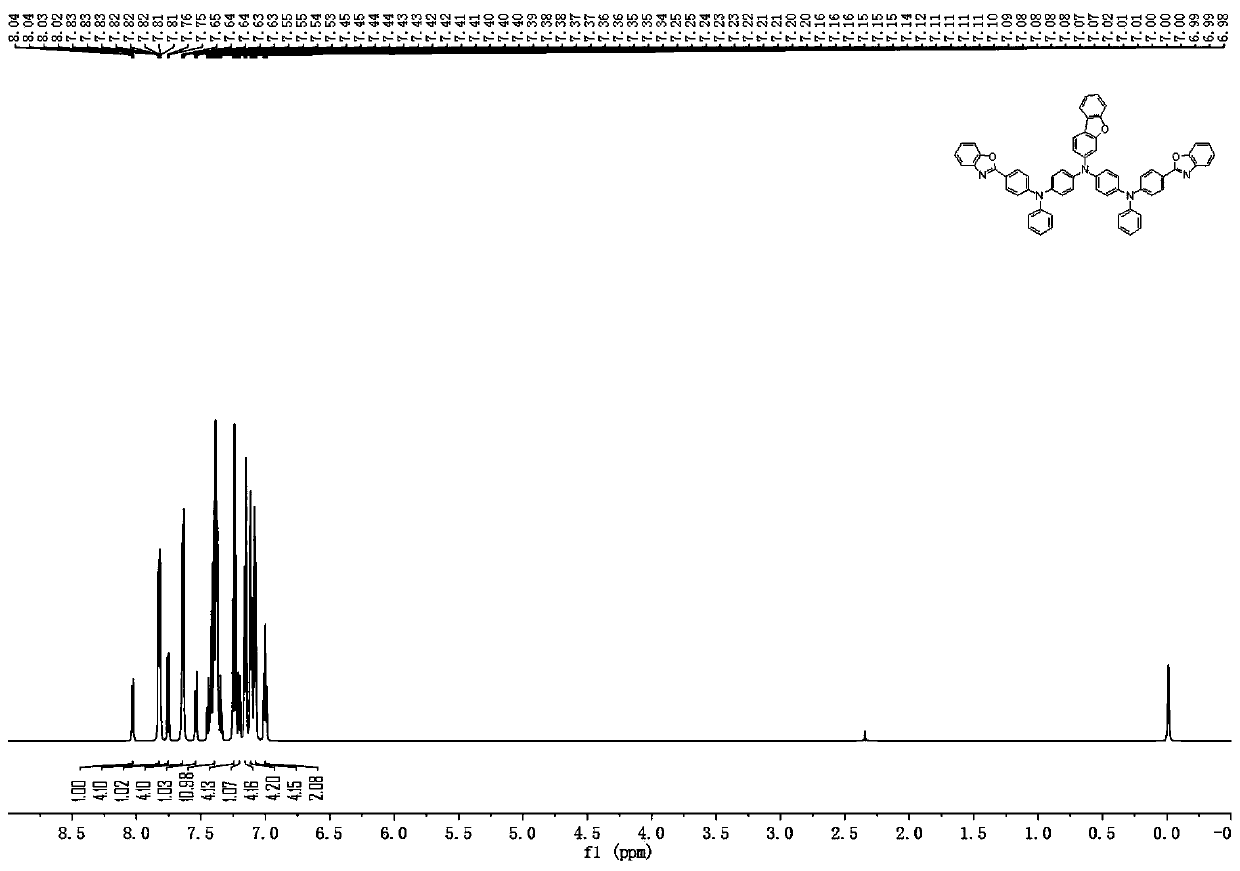
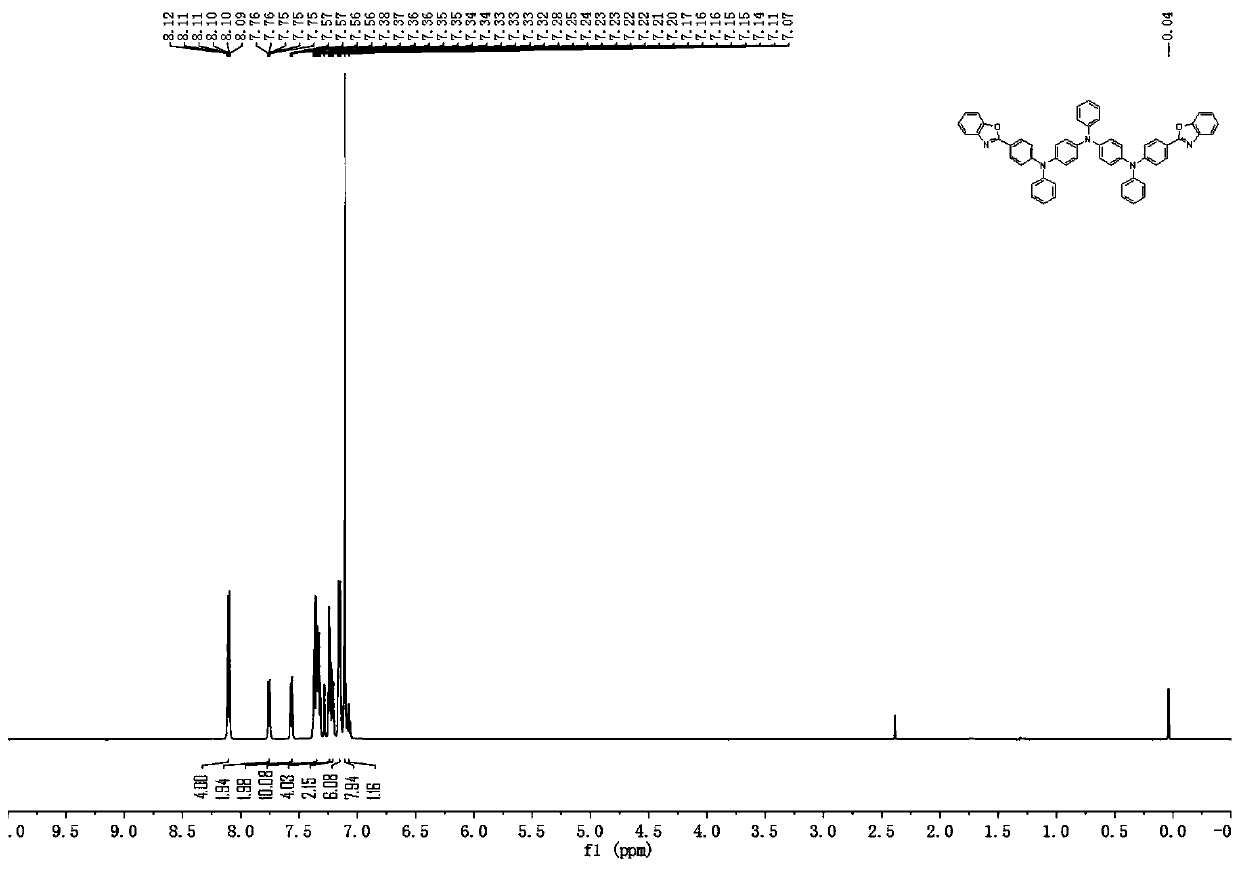
[0104]Description of characterization equipment: mass spectrometry uses AXIMA-CFRplus matrix-assisted laser desorption ionization flight mass spectrometer from Kratos Analytical Company of Shimadzu Group, and chloroform is used as solvent. For elemental analysis, a Vario EL cube organic elemental analyzer from German Elementar Company was used, and the sample mass was 5-10 mg. NMR ( 1 HNMR) use Bruker-510 nuclear magnetic resonance spectrometer (Germany Bruker company), 600MHz, CDCl 3 as solvent and TMS as internal standard.
Synthetic example 1
[0105] Synthesis Example 1: Preparation of Compound 1
[0106]
[0107] (1) Under nitrogen protection, add sodium tert-butoxide (28.8g, 0.30mol), bis(4-bromophenyl)amine (32.7g, 0.10mol), 2-(4-bromobenzene) to a 500ml reaction flask Base) benzoxazole (27.4g, 0.10mol), tris(dibenzylideneacetone) dipalladium (91.5mg, 0.10mmol), toluene (250ml), stirred for 30 minutes, added tri-tert-butylphosphine (0.10mL 1.0 M toluene solution, 0.10 mmol), reacted under reflux conditions for 13 hours. Pour the reaction solution into a 1.5 liter beaker, add toluene (200ml) and water (400ml), stir for 10 minutes, filter with suction, rinse the filter cake with water until the filtrate is neutral, then rinse with toluene and ethanol respectively, and Dry and recrystallize with ethanol to obtain solid intermediate A-1 (42.1 g, yield 81%), and the solid purity is ≧99.9% as detected by HPLC.
[0108] (2) Under nitrogen protection, add 4-(2-benzoxazolyl)aniline (47.3g, 0.225mol), bromobenzene (23...
Synthetic example 2
[0110] Synthesis Example 2: Preparation of Compound 6
[0111]
[0112] The bromobenzene in Synthetic Example 1-(2) was replaced by equimolar 9,9-dimethyl-2-bromofluorene, and the other steps were the same to obtain compound 6 (52.4g, the yield was about 75%), HPLC Detect solid purity ≧99.9%. Mass Spectrum m / z: 1162.23 (calculated: 1163.39). Theoretical element content (%)C 81 h 58 N 6 o 3 : C, 83.63; H, 5.03; N, 7.22; O, 4.13; Measured element content (%): C, 83.12; H, 5.11; N, 7.14; O, 4.25. The above results confirmed that the obtained product was the target product.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com