Fluorescent material for sensitive and selective detection of benzene series and its preparation method and application
A technology of fluorescent materials and benzene series, applied in the field of fluorescent materials, can solve the problems of lack of specificity, slow response, low sensitivity, etc., and achieve the effect of simple operation, fast signal response and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
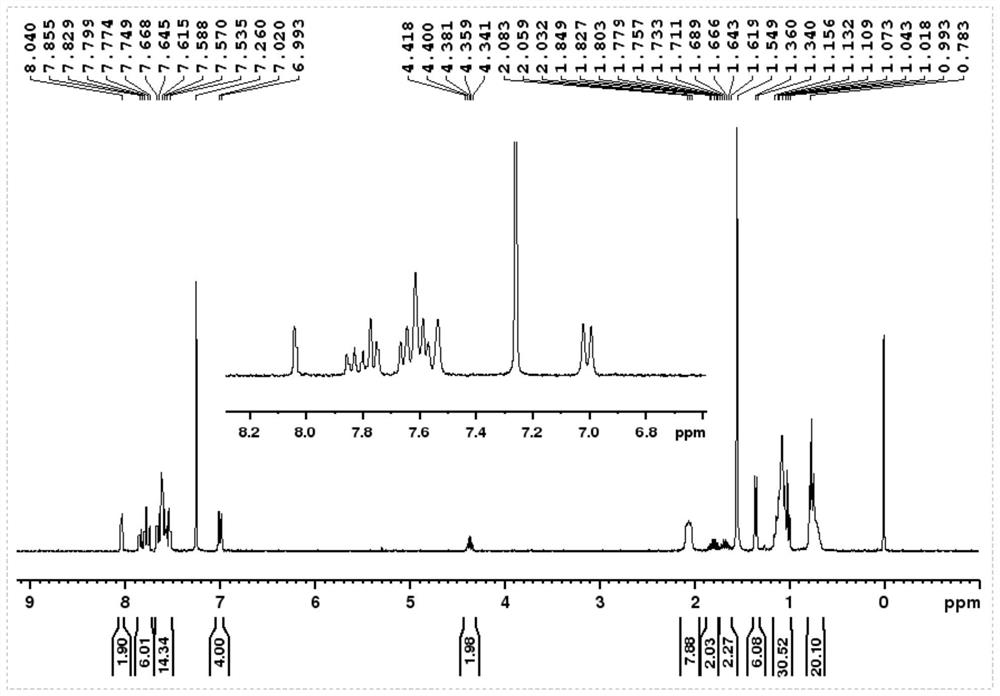
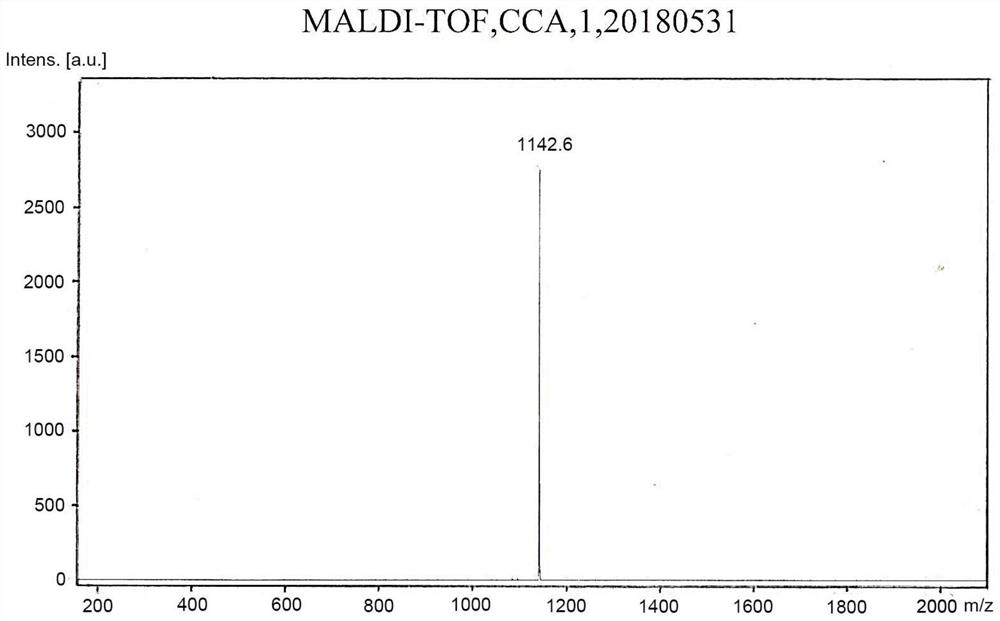
[0120] To prepare compound 1,
[0121]
[0122] (1) Add 2 grams of 4-bromophenol and 1 gram of 2-butanol to 30 ml of tetrahydrofuran, add 3.6 grams of triphenylphosphine, put it in an ice bath after deoxygenation, and then slowly add 2.8 grams of it with a needle Diisopropyl azodicarboxylate, stirred at room temperature for 5 hours, filtered through a gel chromatography column to obtain TM-1;
[0123] (2) Get 1.7 grams of the product obtained in step (1), add 2.3 grams of bis-valeryl diboron, 2.2 grams of potassium acetate and 0.3 grams of 1,1'-bis(diphenylphosphino)ferrocene dichloride Palladium (II), add 30 ml of 1,4-dioxane, react overnight at 80 degrees Celsius after deoxygenation, and obtain TM-2 after filtering through a gel chromatography column;
[0124] (3) 1.1 grams of the product obtained in step (2), 2.3 grams of 9,9-dihexyl-2,7-dibromofluorene, 0.3 grams of tetrakistriphenylphosphine palladium, 1.7 grams of potassium carbonate, and then 20 ml of 1,4-dioxane a...
Embodiment 2
[0137] To prepare compound 2,
[0138]
[0139]
[0140] (1) Add 2 grams of 4-bromophenol and 1 gram of 2-butanol to 30 ml of tetrahydrofuran, add 3.6 grams of triphenylphosphine, put it in an ice bath after deoxygenation, and then slowly add 2.8 grams of it with a needle Diisopropyl azodicarboxylate, stirred at room temperature for 5 hours, filtered through a gel chromatography column to obtain TM-1;
[0141] (2) Get 1.7 grams of the product obtained in step (1), add 2.3 grams of bis-valeryl diboron, 2.2 grams of potassium acetate and 0.3 grams of 1,1'-bis(diphenylphosphino)ferrocene dichloride Palladium (II), add 30 ml of 1,4-dioxane, react overnight at 80 degrees Celsius after deoxygenation, and obtain TM-2 after filtering through a gel chromatography column;
[0142] (3) 1.1 grams of the product obtained in step (2), 2.3 grams of 9,9-dihexyl-2,7-dibromofluorene, 0.3 grams of tetrakistriphenylphosphine palladium, 1.7 grams of potassium carbonate, and then 20 ml of 1...
Embodiment 3
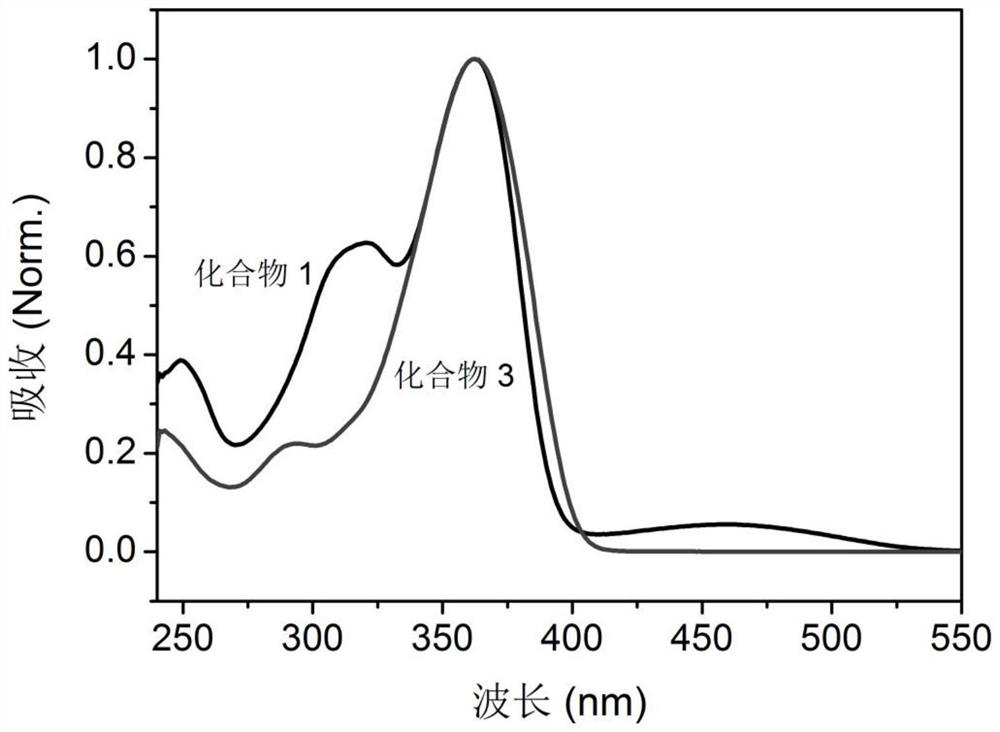
[0150] Compound 3 having the following molecular formula was prepared.
[0151]
[0152] (1) Add 2 grams of 4-bromophenol and 1 gram of 2-butanol to 30 ml of tetrahydrofuran, add 3.6 grams of triphenylphosphine, put it in an ice bath after deoxygenation, and then slowly add 2.8 grams of it with a needle Diisopropyl azodicarboxylate, stirred at room temperature for 5 hours, filtered through a gel chromatography column to obtain TM-1;
[0153] (2) Get 1.7 grams of the product obtained in step (1), add 2.3 grams of bis-valeryl diboron, 2.2 grams of potassium acetate and 0.3 grams of 1,1'-bis(diphenylphosphino)ferrocene dichloride Palladium (II), add 30 ml of 1,4-dioxane, react overnight at 80 degrees Celsius after deoxygenation, and obtain TM-2 after filtering through a gel chromatography column;
[0154](3) 1.1 grams of the product obtained in step (2), 2.3 grams of 9,9-dihexyl-2,7-dibromofluorene, 0.3 grams of tetrakistriphenylphosphine palladium, 1.7 grams of potassium car...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com