Trophoblast, preparation method thereof and application of trophoblast in massive rapid amplification of gamma delta T cells
A technology of nourishing cells and cells, which is applied in the fields of genetic engineering and cell biology, can solve the problems of low content and low immunogenicity of γδT cells, and achieve the effect of reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
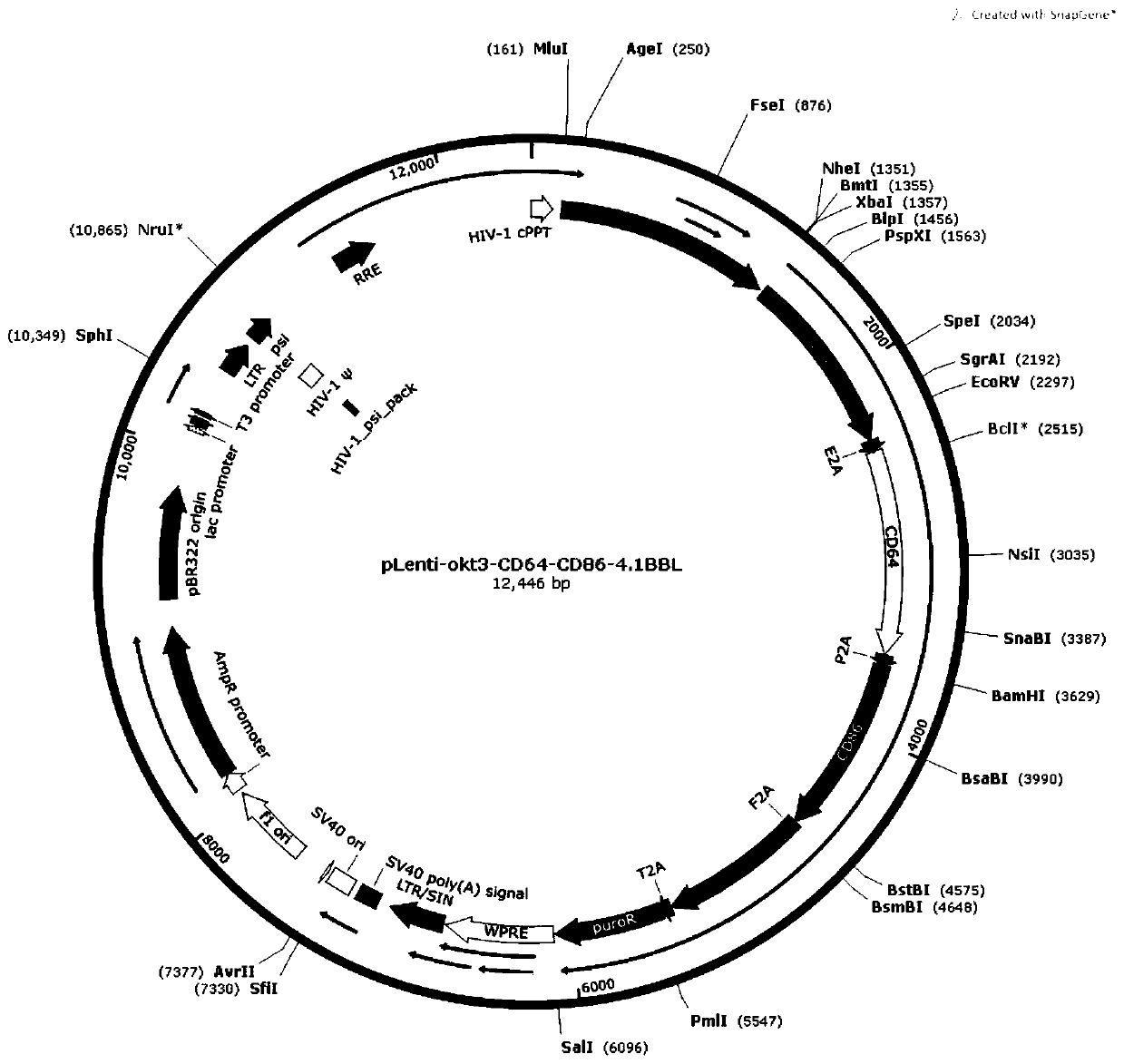
[0074] Construction of pLenti-okt3-CD64-CD86-4.1BBL lentiviral plasmid vector
[0075] Query on websites such as NCBI to obtain okt3 (as shown in SEQ IDNO:1), CD64 (as shown in SEQ IDNO:2), CD86 (as shown in SEQ IDNO:3), 4.1BBL (as shown in SEQ IDNO:4) The whole gene coding sequence;
[0076] The okt3-E2A-CD64-P2A-CD86-F2A-4.1BBL-T2A-Puro gene was synthesized by gene synthesis, and two restriction sites XbaI and SalI were synthesized on both sides of it, and the coding region avoided these two Restriction sites;
[0077] The above synthesized gene was double digested with XbaI and SalI enzymes, and then connected to the lentiviral vector pLenti-mbIL21-4.1BBL (gifted by the Institute of Genetics and Development, Chinese Academy of Sciences) by T4 DNA ligase to obtain pLenti-okt3-CD64- CD86-4.1BBL (as shown in SEQ ID NO: 5), such as figure 1 shown.
[0078] The ligation product was transformed into E.coli (DH5α) cells, and the positive clones were identified by PCR, and then...
Embodiment 2
[0081] Packaging preparation of recombinant lentivirus
[0082] 1. Add 4.5million 293FT cells and 9ml DMEM complete medium to each 10cm cell culture dish, mix well, and culture in a cell culture incubator (37°C, 5% (v / v) CO 2 );
[0083] 2. On the second day of culture, add the following reagents to each culture dish: 500 μL jetPRIME buffer, 6 μg pLenti-okt3-CD64-CD86-4.1BBL or pLenti-CD64-CD86-4.1BBL plasmid vector, 3 μg psPAX2 and 1.5 μg pMD2.G , mix evenly, then add jetPRIME, 25μL / 10cm petri dish, mix evenly again, and let stand at room temperature for 10min to get the mixed solution;
[0084] 3. Take out the 293FT cells used for packaging the virus from the incubator, add the mixture evenly to each culture dish, mix well, and put it into the incubator to continue culturing (37°C, 5% (v / v) CO 2 ). After culturing for 4 h, discard the old medium, add PBS to wash the cells, then add DMEM complete medium containing 10wt% FBS, and put them in an incubator for cultivation (37...
Embodiment 3
[0088] Preparation of K562-okt3-CD64-CD86-4.1BBL trophoblast cells
[0089] Put 1 million vigorously growing K562 cells into one well of a 24-well plate for culture, and the medium is 1 ml of 1640 containing 10% FBS;
[0090] Then add 300 μL of lentiviral particles concentrated by ultracentrifugation of the above-mentioned 30 ml of virus supernatant into the wells, and culture (37° C., 5% (v / v) CO 2 ) After 5 days, selective culture was carried out with 3 μg / ml of Puromycin;
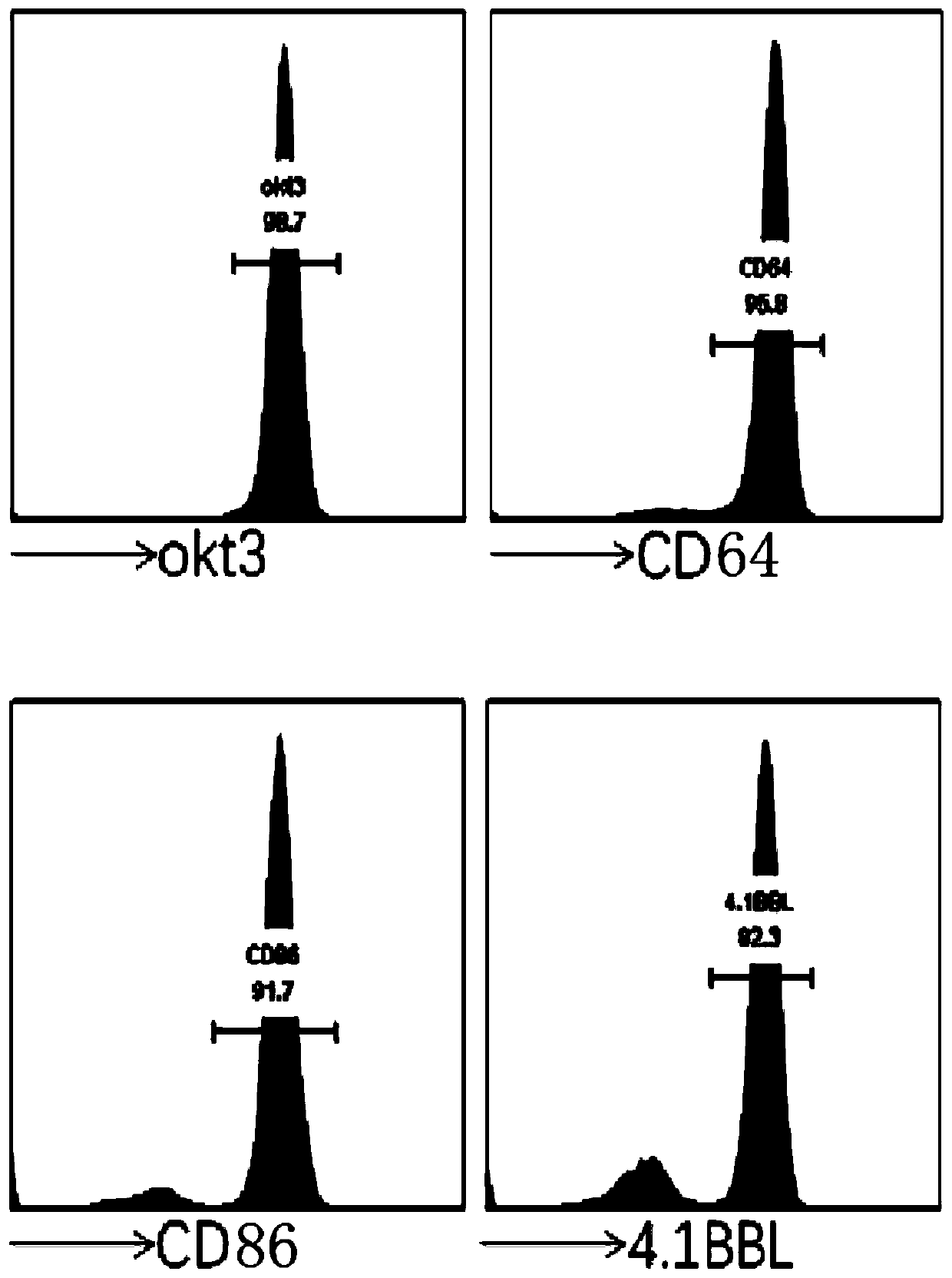
[0091]Detect the expression of these four genes okt3, D64, D86 and 4.1BBL in K562 cells by flow cytometry, so that the expression of each gene is above 80% (see image 3 );
[0092] Then 100Gy of γ-rays were used to irradiate for 10 minutes, and after irradiation, branches were frozen in a -80°C refrigerator for future γδT cell culture experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com