Hemicyanine compound based on benzothiazole biheterocycle, and preparation method and application thereof
A compound and alkylation reaction technology, applied in chemical instruments and methods, organic chemistry, instruments, etc., can solve the problems of unable to meet the research needs of scientific researchers, complex synthesis process, insufficient number of probes, etc., to achieve easy promotion, Effect of low cytotoxicity and high quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
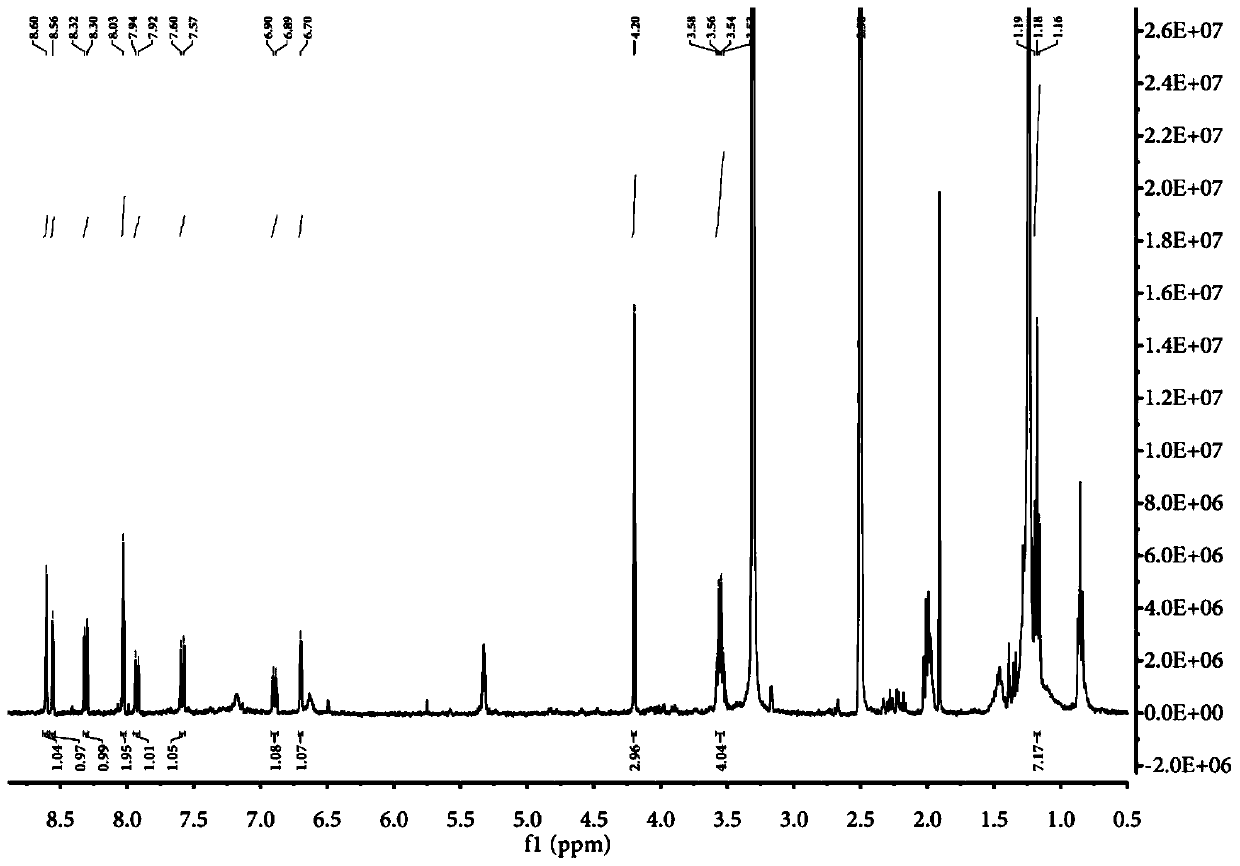
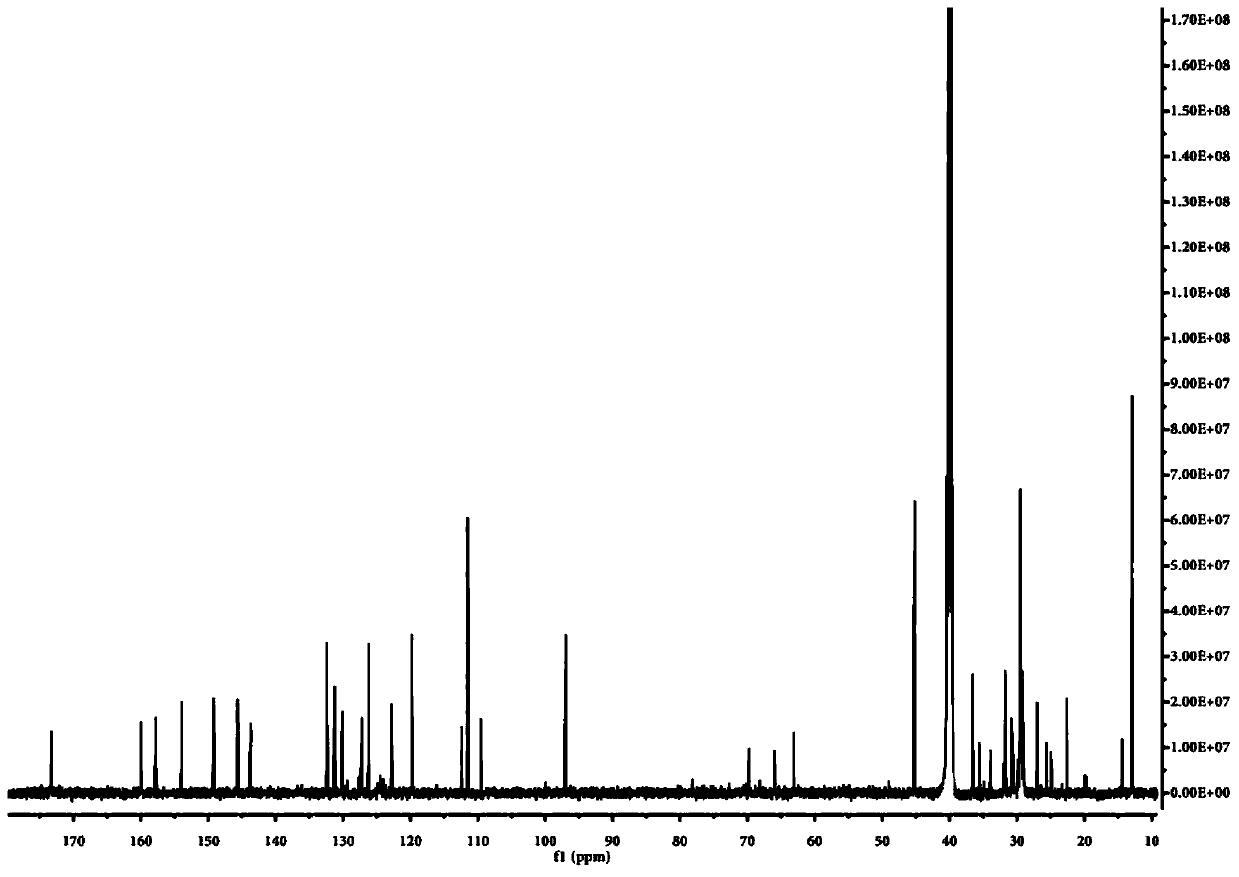
[0078] Embodiment 1, the preparation of the compound represented by formula A and the specific recognition of nucleic acid double-stranded structure by fluorescent probe
[0079] One, the synthesis of the described probe of formula A
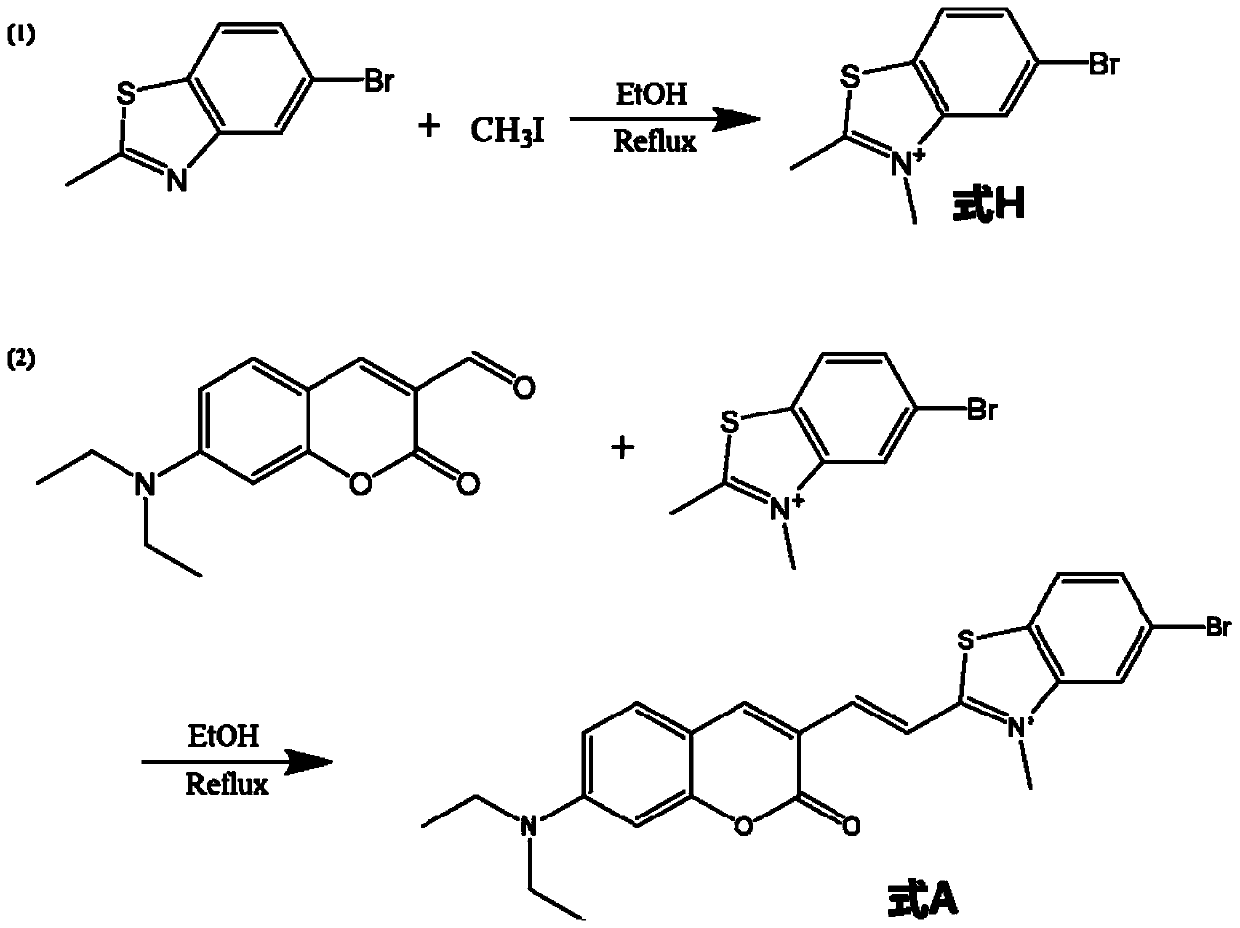
[0080] like figure 1 As shown, it is synthesized according to the following steps:
[0081] 1. Add 227mg (1.0mmol) of 5-bromo-2-methylbenzothiazole into a 25mL single-necked round bottom flask filled with 10mL of ethanol. After preheating at 78-85°C for 5 minutes, add 124.5 μL (2.0 mmol) methyl iodide, continue to heat and reflux for 12 hours, and stop when the solution turns from light yellow transparent to orange yellow. After cooling to room temperature, distill under reduced pressure to obtain the molecule of formula H.
[0082]
[0083] 2. Add 36.3 mg (0.15 mmol) of the molecule of formula H into a 10 mL single-necked round bottom flask filled with 2 mL of ethanol. After preheating at 78-85°C for 5 minutes, add 24.5 mg (0.1 mmol) of ...
Embodiment 2
[0108] Embodiment 2, the fluorescent probe described in formula B specifically recognizes the nucleic acid double-stranded structure
[0109] One, the synthesis of the described probe of formula B
[0110] 36.3 mg (0.15 mmol) of the molecule of formula H was added to a 10 mL single necked round bottom flask containing 2 mL of ethanol. After preheating at 78-85°C for 5 minutes, add 27.3mg (0.1mmol) of 5-bromo-2,2'-bithiophene-5'-carboxaldehyde, continue heating and reflux for 12 hours, the solution turns from light yellow to deep red, then stop, cool After reaching room temperature, it was distilled under reduced pressure to obtain the crude product of formula B.
[0111]
[0112] Crude product B was dissolved in dichloromethane and chromatographed on silica gel (CH 2 Cl 2 / MeOH, v / v, 30:1) to obtain the compound as an orange-red solid formula B molecule (32 mg, 64.4% yield). Then, the compound was further purified by high performance liquid chromatography. The separati...
Embodiment 3
[0121] Embodiment 3, the fluorescent probe described in formula C specifically recognizes the nucleic acid G-quadruplex structure
[0122] One, the synthesis of the described probe of formula C
[0123] 36.3 mg (0.15 mmol) of the molecule of formula H was added to a 10 mL single necked round bottom flask containing 2 mL of ethanol. After preheating at 78-85°C for 5 minutes, add 22.3mg (0.1mmol) N-ethylcarbazole-3-carboxaldehyde, continue to heat and reflux for 12 hours, the solution turns from light yellow to orange red and then stops, cool to room temperature and distill under reduced pressure , to obtain the crude product of formula C.
[0124] The crude product C was dissolved in dichloromethane and chromatographed on silica gel (CH 2 Cl 2 / MeOH, v / v, 30:1) to obtain the compound as an orange-red solid formula C molecule (39 mg, 87.1% yield). Then, the compound was further purified by high performance liquid chromatography. The separation and purification steps of high...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com