A method for preparing all-trans vitamin A acetate
A vitamin and acetate technology, applied in organic chemistry methods, chemical instruments and methods, physical/chemical process catalysts, etc., can solve problems such as poor catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
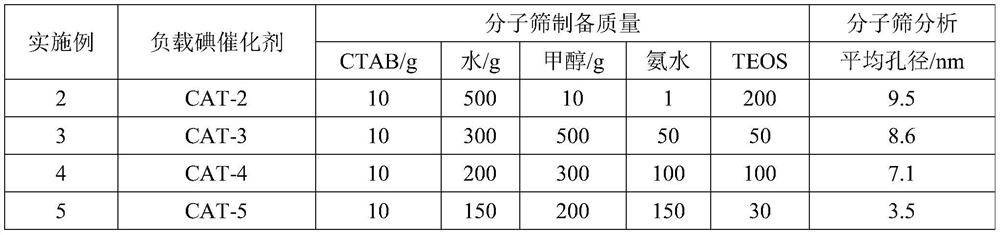
[0041] Molecular sieve preparation: Take 10g of cetyltrimethylammonium bromide (CTAB) and add it to 120g of pure water, then add 1000g of anhydrous methanol and 200g of concentrated ammonia water respectively, after ultrasonication at room temperature for 30min, add 20g of ethyl orthosilicate dropwise (TEOS), after ultrasonication at room temperature for 30 minutes, suction filtration and washing with pure water until pH=7, drying at 120°C for 100 minutes and grinding into powder, and then roasting in a muffle furnace at 700°C for 6 hours to obtain MCM-48 Molecular sieve. The specific surface area and pore diameter analyzer of the American Quanta Company was used to measure, and the average pore diameter of the molecular sieve prepared by the above method was about 2.6 nm through BJH analysis.
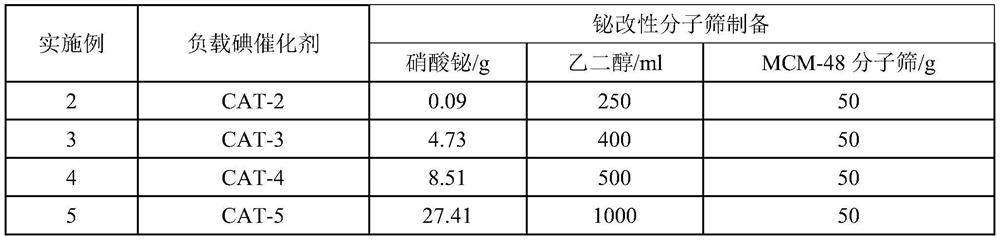
[0042] Preparation of bismuth-modified molecular sieve: Add 0.05g of bismuth nitrate to 100ml of ethylene glycol, stir until completely dissolved, immerse 50g of the prepared molecular s...
Embodiment 2-5
[0046] The preparation process of catalysts CAT2-5 is the same as that of catalyst CAT-1, and the ratio of each material is shown in the following tables 1-3;
[0047] Table 1. Molecular sieve preparation feed list
[0048]
[0049] Table 2. Bismuth modified molecular sieve preparation feed list
[0050]
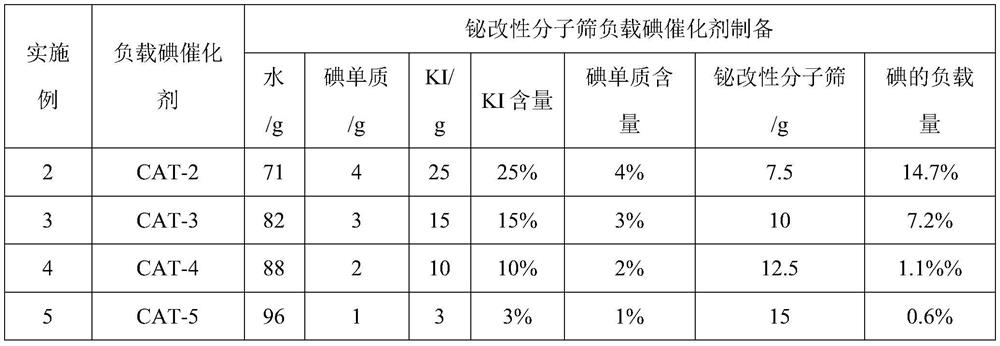
[0051] Table 3. Bismuth-modified molecular sieve supported iodine catalyst preparation feed list
[0052]
[0053] The isomerization reaction conditions of embodiment 2-5 are shown in Table 4, and the results are shown in Table 5
[0054] Table 4. Isomerization reaction conditions
[0055]
[0056] Table 5. Embodiment 1~5 isomerization experiment result
[0057]
Embodiment 6
[0059] Catalyst life test:
[0060] The preparation of all-trans vitamin A acetate: take by weighing 100gVA crude oil (11-cis isomer content wt23% in the crude oil, 9-cis isomer content 27wt%, all-trans isomer content 35% ) and 400g n-hexane are mixed and prepared into VA crude oil mass fraction as 20% n-hexane solution, add the catalyst CAT-2 prepared in 0.1g embodiment 2, after the reaction system feeds nitrogen replacement 10min, stir reaction at 50 ℃ 3h, the composition of the reaction solution was analyzed by high performance liquid chromatography. After the reaction, the reaction solution was filtered with a sand core funnel, the catalyst was washed with fresh acetone, and the solid catalyst obtained after drying was subjected to the next batch of isomerization experiments. The reaction conditions were the same, and a total of 5 batches were applied. The results are listed in the table 6.
[0061] Table 6. Example 6 isomerization experimental results
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com