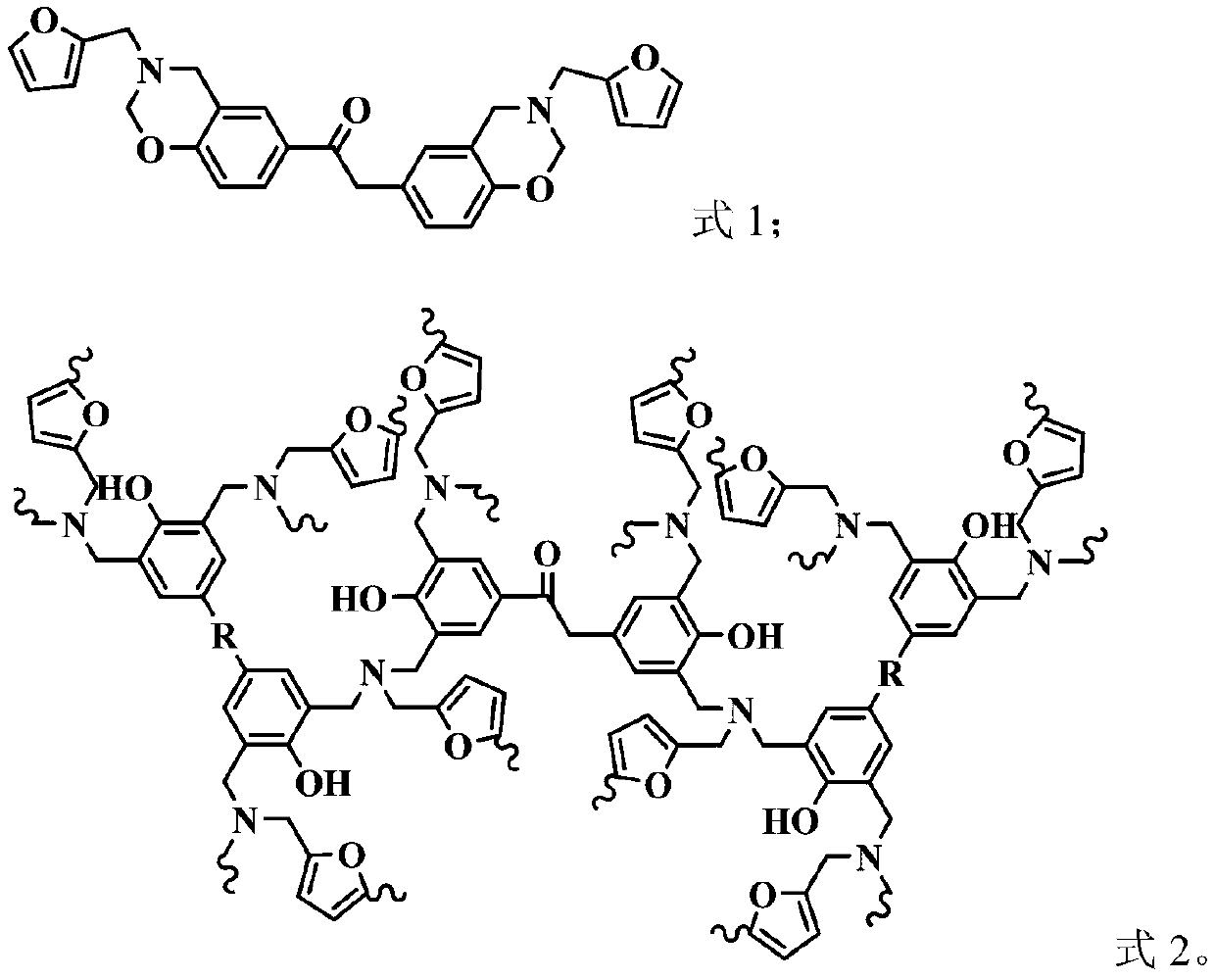
Benzoxazine intrinsic flame-retardant resin and preparation method thereof
A benzoxazine and intrinsic flame retardant technology, applied in the field of materials, can solve the problems of unknown flame retardant properties of resins, unreliable experimental results, etc., and achieve improved glass transition temperature and thermal stability, low limiting oxygen Index, the effect of saving oil resources
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation of deoxybenzoin bisphenol:
[0044] Add deoxy-p-anizoin and pyridinium hydrochloride into the reaction vessel, wherein the molar ratio of deoxy-p-anizoin to pyridinium hydrochloride is 1:4, react at 200°C for 5 hours, and pour the reaction solution into glacial acetic acid after the reaction Recrystallize in an aqueous solution to obtain white crystals, and obtain deoxybenzoin bisphenol powder after drying.
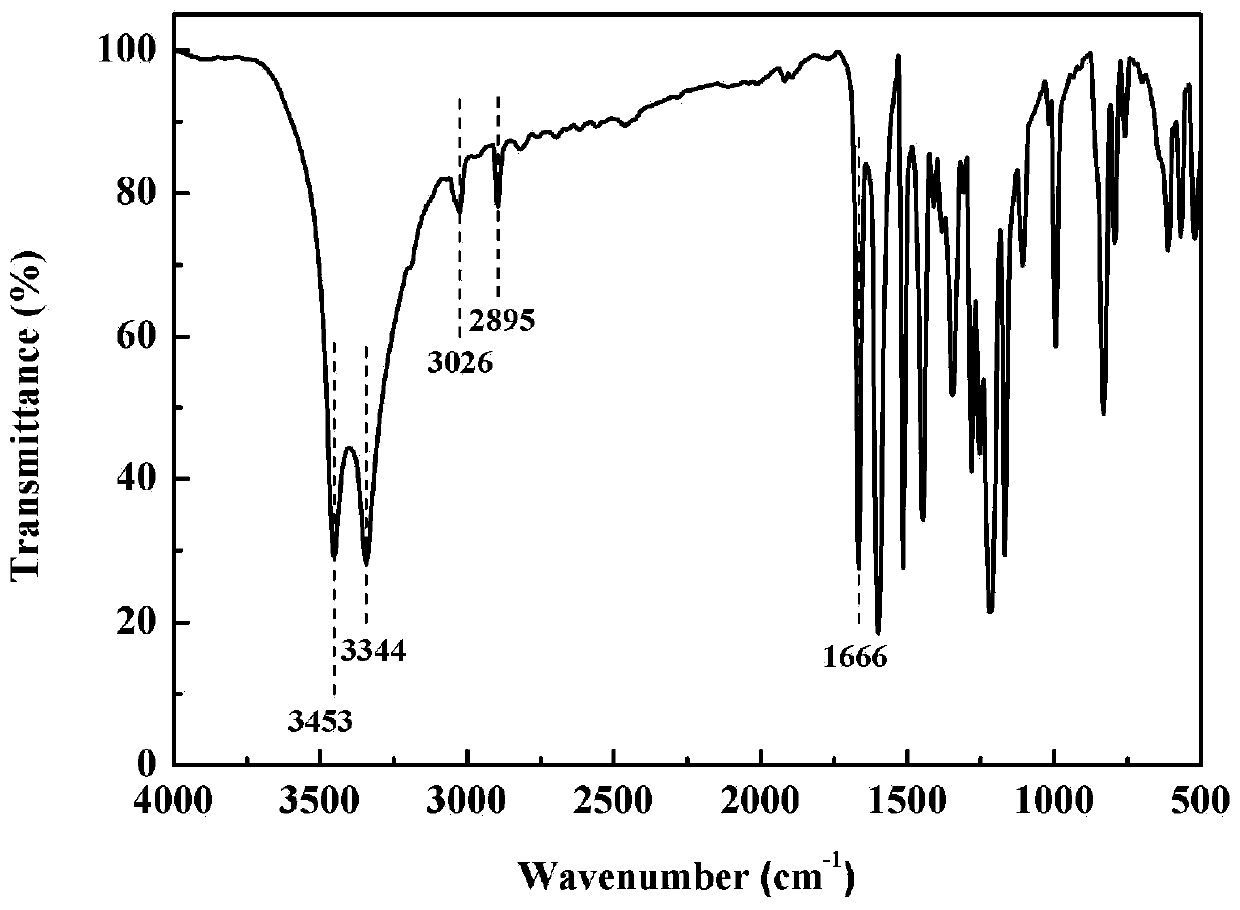
[0045] Such as figure 1 Shown is the infrared spectrogram of the deoxybenzoin bisphenol prepared in this embodiment, 3453, 3344cm -1 Represents the characteristic absorption peak of phenol-OH, 1666cm -1 Represents the characteristic absorption peak of C=O, 3026cm -1 The position is the stretching vibration peak of C-H on the benzene ring, 2895cm -1 at -CH 2 stretching vibration peak. To sum up, it shows that the synthesized product of this example is deoxybenzoin bisphenol, and its molecular structure is shown in formula 3.
Embodiment 2
[0047] Preparation of intrinsic flame retardant benzoxazine monomer:
[0048] 5.70g (0.025mol) deoxybenzoin bisphenol, 4.85g (0.05mol) furfurylamine, 13.50g (0.45mol) paraformaldehyde prepared in Example 1 are added into the condensing tube, magnetic stirring, thermometer In a 250mL three-necked flask, the molar ratio of phenolic hydroxyl group, amino group and aldehyde functional group is 1:1:9, then add 75mL tetrahydrofuran solvent, mix well and heat to 65°C for 130h, after the reaction, pour the reaction solution into 100mL methanol Precipitate in the solution to obtain a milky white suspension, let it stand for 12 hours, remove the supernatant to obtain a brown precipitate, dry the brown precipitate at 60°C for 8 hours in vacuum, and finally grind the dried product to obtain a brown powder, which is intrinsic flame retardant type benzoxazine monomer.
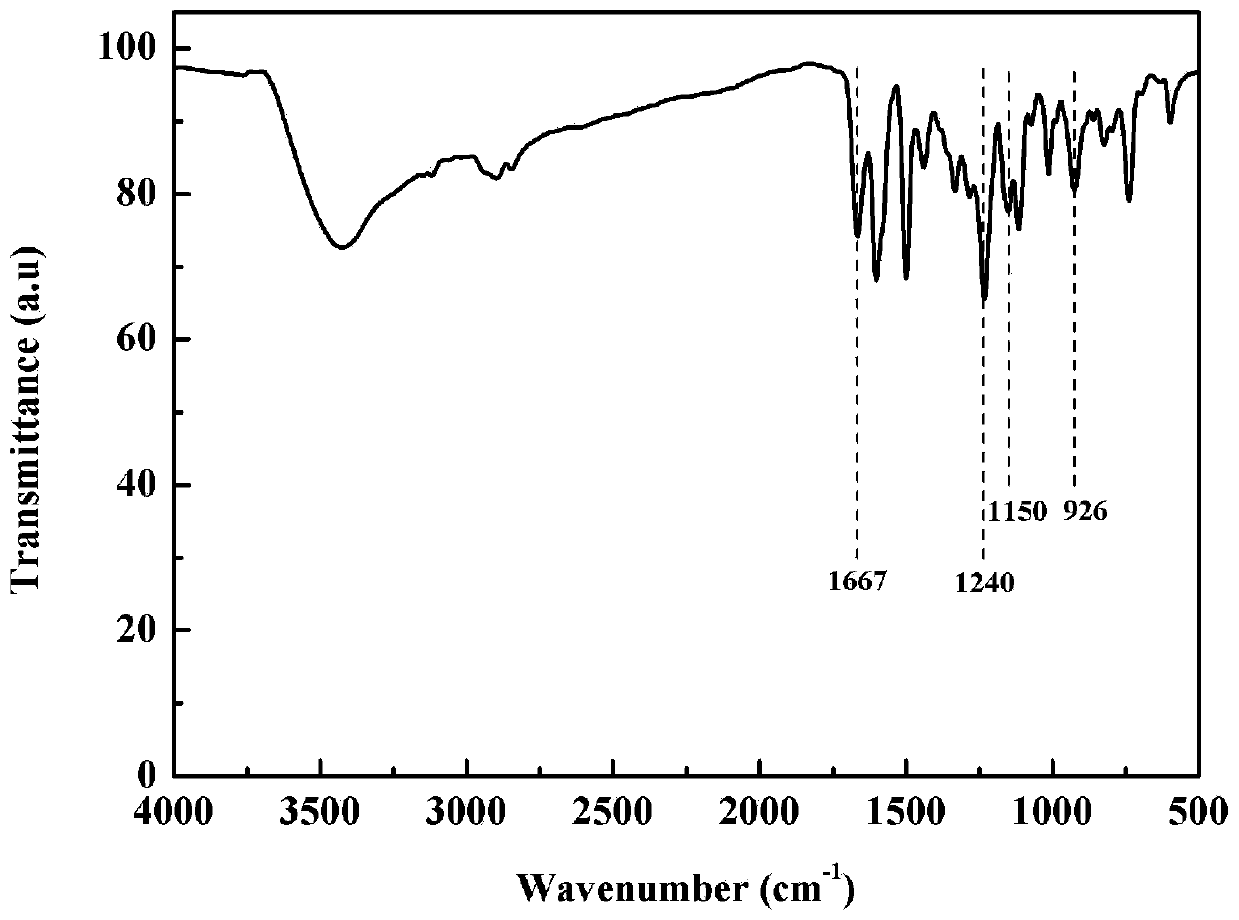
[0049] Such as figure 2 Shown is the infrared spectrogram of the intrinsic flame retardant benzoxazine monomer prepared i...
Embodiment 3
[0051] Preparation of intrinsic flame retardant benzoxazine monomer:
[0052] 68.4g (0.3mol) deoxybenzoin bisphenol, 29.1g (0.3mol) furfurylamine, 36.0g (1.20mol) paraformaldehyde prepared in Example 1 are added to be equipped with condenser tube, magnetic sub-stirring, thermometer In a 250mL three-necked flask, the molar ratio of phenolic hydroxyl group, amino group and aldehyde functional group is 6:3:12, then add 75mL of toluene solvent, mix well, heat to 115°C for 8 hours, and pour the reaction solution into 100mL methanol after the reaction Precipitate in the solution to obtain a milky white suspension, let it stand for 12 hours, remove the supernatant to obtain a brown precipitate, dry the brown precipitate at 60°C for 8 hours in vacuum, and finally grind the dried product to obtain a brown powder, which is the intrinsic flame retardant type benzoxazine monomer.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com