Lithium ion battery electrolyte suitable for quick charging and lithium ion battery
An electrolyte and lithium salt technology, applied in the field of lithium-ion battery materials, can solve the problems of long charging time and poor low-temperature performance, and achieve the effects of ensuring fast delivery, reducing charging impedance, and inhibiting dissolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0044] The preparation method of the present invention will be further described in detail in conjunction with specific examples below. It should be understood that the following examples are only for illustrating and explaining the present invention, and should not be construed as limiting the protection scope of the present invention. All technologies realized based on the above contents of the present invention are covered within the scope of protection intended by the present invention.
[0045] The experimental methods used in the following examples are conventional methods unless otherwise specified; the reagents and materials used in the following examples can be obtained from commercial sources unless otherwise specified.
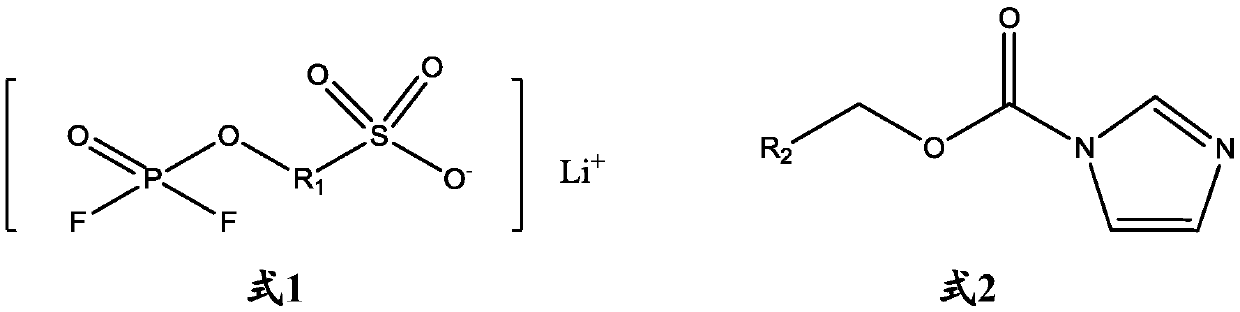
[0046] The structure of the lithium salt compound shown in formula 1 involved in the following examples and comparative examples is as follows:
[0047]
[0048] The structure of the imidazole carboxylate compounds shown in formula 2 involved in...
Embodiment 1
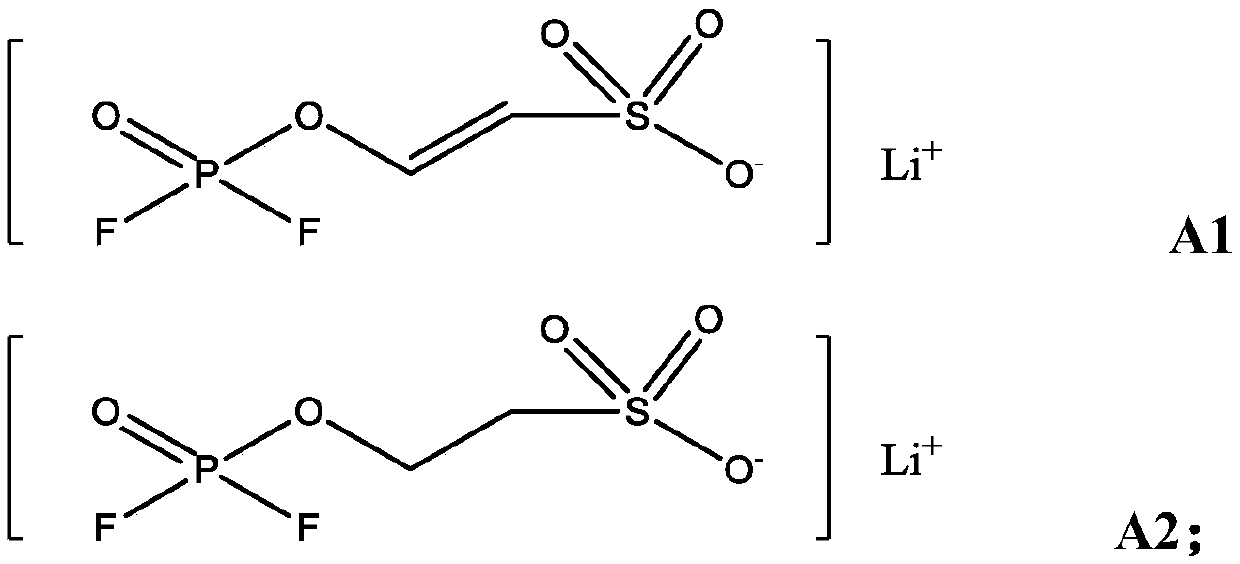
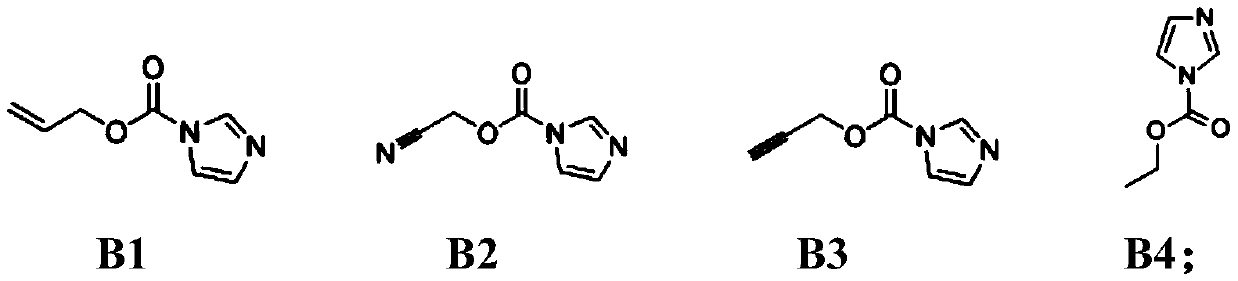
[0051] Mix the solvent ethylene carbonate / propylene carbonate / diethyl carbonate / propyl propionate in a mass ratio of 20:15:20:45, and add 0.5% of A1 and 0.8% of B1 and 0.5% LiPF 2 o 2 As an additive, 1 mol / L lithium hexafluorophosphate was finally added to obtain the electrolyte solution of Example 1.
[0052] The electrolyte solution was injected into the non-liquid-injected cell comprising the positive electrode sheet, the negative electrode sheet and the separator to make a lithium ion battery, and the battery of Example 1 was obtained.
Embodiment 2-13 and comparative example 1-6
[0054] The preparation method of other embodiment and comparative example is the same as embodiment 1, and difference only is that the material of each component is different, specifically as shown in table 1 below:
[0055] The composition of the electrolytic solution of table 1 embodiment 1-13 and comparative example 1-6
[0056]
[0057] Electrochemical performance test is carried out to the lithium ion battery of above comparative example and embodiment gain:
[0058] 25°C normal temperature cycle test: put the batteries obtained in Examples 1-13 and Comparative Examples 1-6 in an environment of (25±2)°C, and let them stand for 2-3 hours until the battery body reaches (25±2)°C , the battery is charged according to 5C constant current with a cut-off current of 0.05C. After the battery is fully charged, it is left for 5 minutes, and then discharged at a constant current of 1C to a cut-off voltage of 3.0V. Record the highest discharge capacity of the first 3 cycles as the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com