Device and method for decomposing chlorate generated by side reaction of anode chamber of electrolytic cell in chlor-alkali production and related acid adding process
A chlorate and electrolytic cell technology, applied in electrolysis process, electrolysis components, chlorine/hydrogen chloride, etc., can solve problems such as long decomposition time, complicated operation, and low decomposition rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
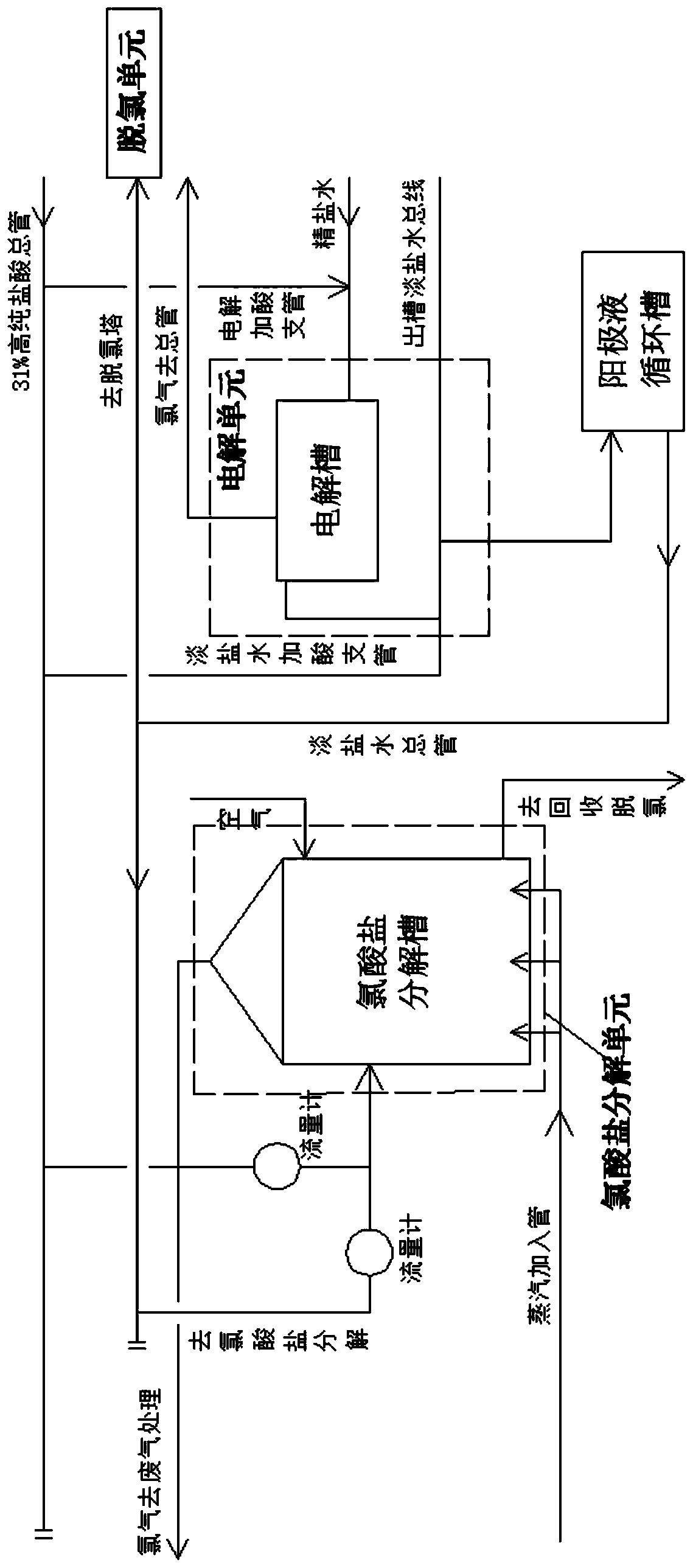
[0100] combined with figure 1 , 2 , the invention provides an acid addition process involved in the by-product chlorate in the anode chamber of the electrolytic cell in chlor-alkali production. The acid addition method in the electrolysis production process is divided into an electrolysis unit, a dechlorination unit, and a chlorate unit by a high-purity hydrochloric acid main pipe. Hydrochloric acid is added to the decomposition unit at the same time to solve the migration of OH in the anode chamber of the electrolytic cell alone - , dechlorinated light brine with high pH value, hydrochloric acid for chlorate decomposition,
[0101] Change to combine the original chlorate decomposition, dechlorination and part of the acid used for electrolysis, add high-purity hydrochloric acid from the chlorate decomposition place, and add the light brine containing by-product chlorate that is diverted from the chlorate decomposition place Mix to achieve chlorate decomposition. After the ch...
Embodiment 2
[0109] combined with figure 2 , the invention provides a kind of decomposing device of the chlorate that the side reaction of anode compartment of electrolyzer generates in a kind of chlor-alkali production, comprises:
[0110] The electrolytic cell is used for chlor-alkali production. The refined brine pipelines connected to the electrolytic cell, the light brine pipelines out of the tank, and the light brine pipelines out of the tank are connected to the light brine main pipe through the anolyte circulation tank;
[0111] High-purity hydrochloric acid main pipe, the high-purity hydrochloric acid main pipe is divided into two branches, the electrolytic acid adding branch pipe goes to the electrolytic tank into the refined brine pipeline, and the second branch pipe goes to the chlorate decomposition tank;
[0112] The chlorate decomposition tank provides a place for the decomposition of by-product chlorate in chlor-alkali production. The chlorate decomposition tank is connect...
Embodiment 3
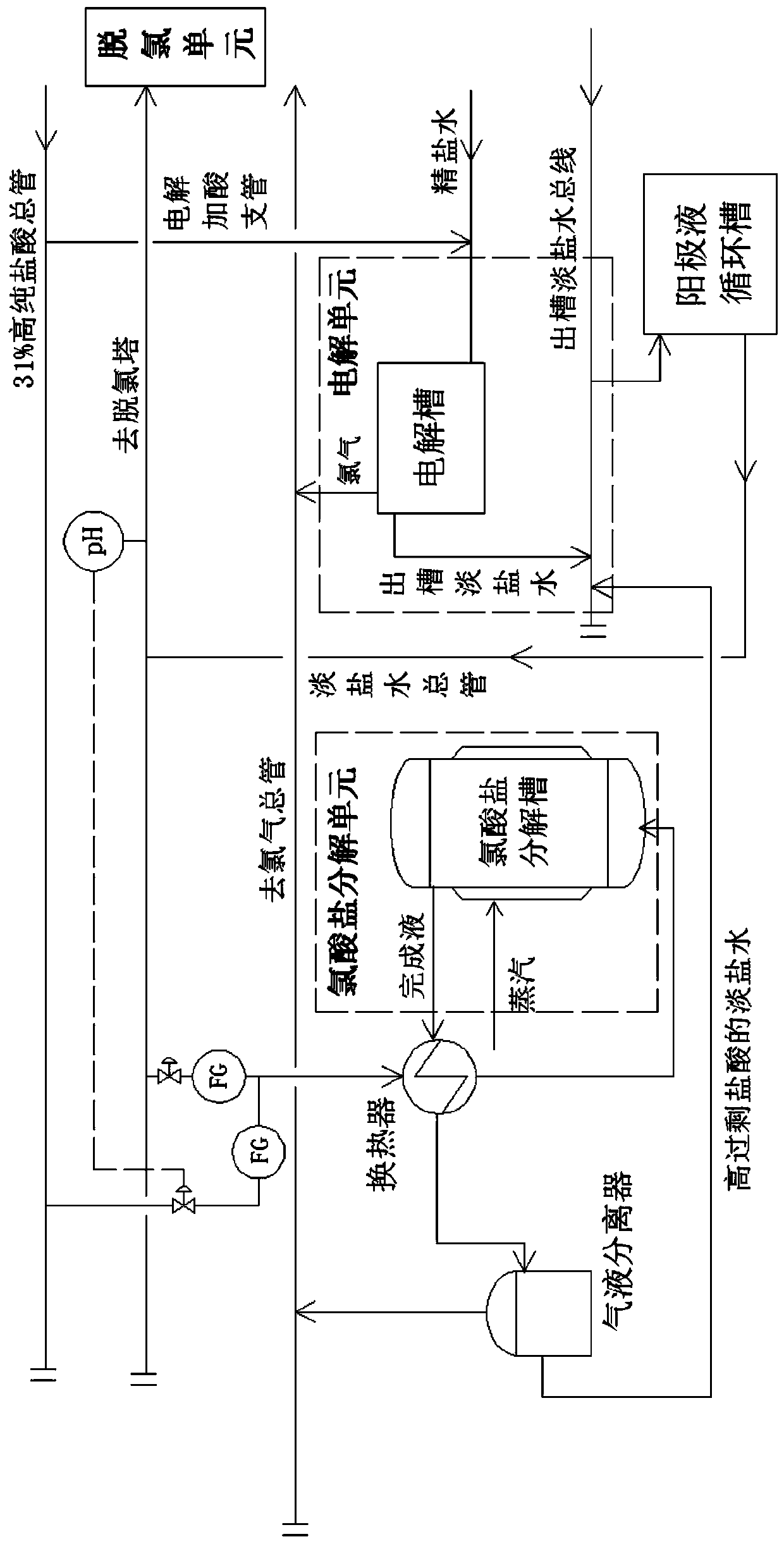
[0118] combined with figure 2 , the present invention provides a method for decomposing chlorate generated by side reactions in the anode chamber of an electrolytic cell in chlor-alkali production, based on the decomposition device of Embodiment 2, the method is based on the content of by-product chlorate contained in chlor-alkali production, from the anode The corresponding light brine is diverted from the liquid circulation tank to the chlorate decomposition place, and high-purity hydrochloric acid is concentratedly added to the chlorate decomposition place according to the high superacid amount to realize the chlorate decomposition;
[0119] After the chlorate decomposition is completed, the light brine with high excess hydrochloric acid returns to the anolyte circulation tank to replace the high-purity hydrochloric acid to reduce the pH value of the circulating anolyte. It is sent to the units of electrolysis, dechlorination and chlorate decomposition. The electrolytic ce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com