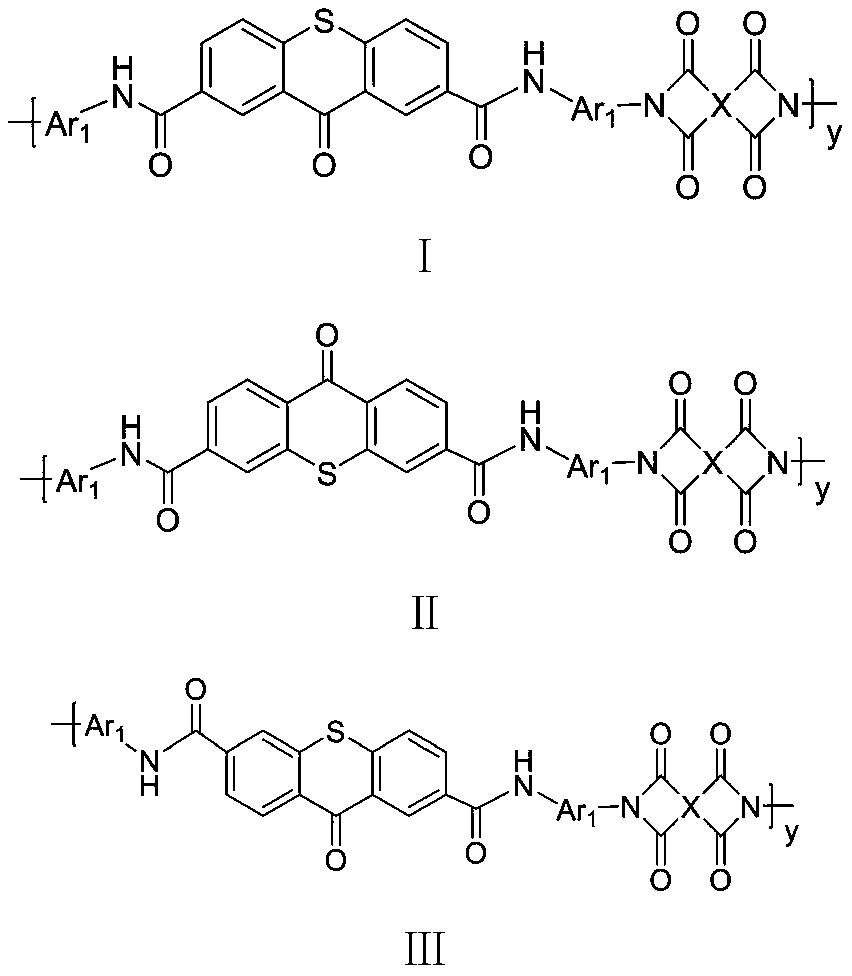
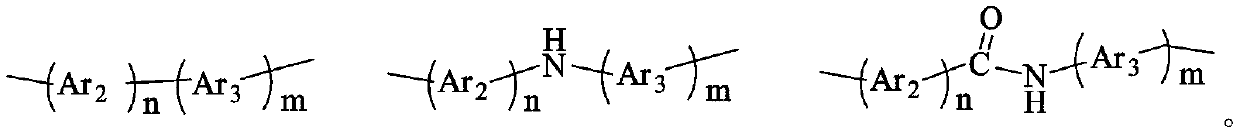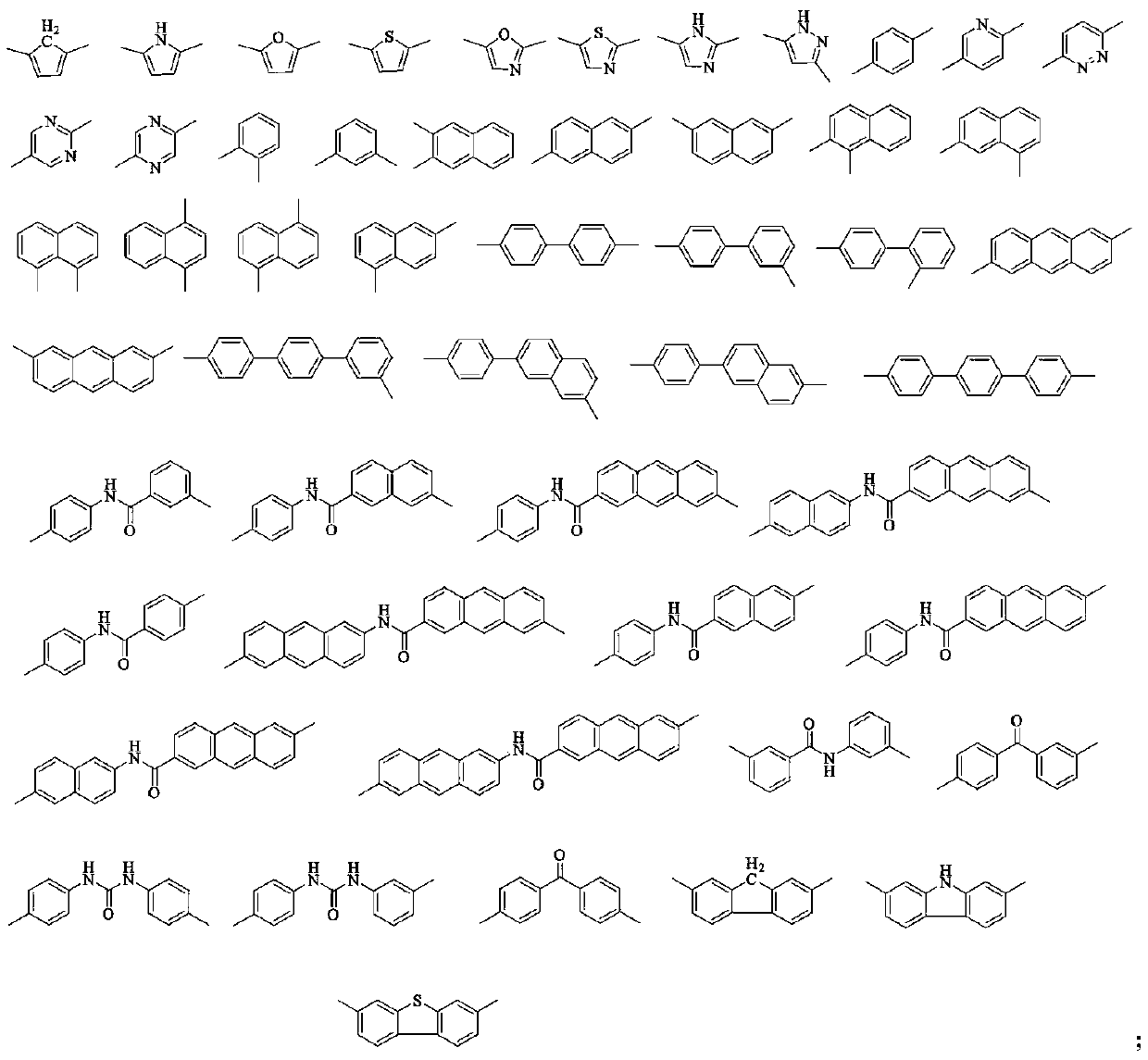Thioxanthone polyimide with high planar rigidity as well as preparation method and application thereof
A polyimide and thioxanthone technology, which is applied in the field of material science, can solve the problems of poor heat resistance of high-barrier film, influence of flexibility of coating film, easy to break and fall off, etc., and achieve excellent barrier performance and regular molecular chain arrangement. , the effect of high electron density
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] This example provides
[0044] Synthesis of N3,N6-bis(4-aminophenyl)-9-oxo-9H-thioxanthene-3,6-dicarboxamide:
[0045]
[0046] S1. Synthesis of intermediate 9-oxo-9H-thioxanthene-3,6-dicarbonitrile:
[0047] Add 3.70g (0.01mol) of 3,6-dibromo-9H-thioxanthen-9-one, 4.478g (0.05mol) of cuprous cyanide, and 50ml of dry NMP into a 500ml three-necked flask, reflux at 140°C for 24h, and then Will H 2 O (180mL), HCl (60mL) and FeCl3 (4.19g, 25.8mmol) were poured into the reaction solution and stirred for 1h, cooled to room temperature, filtered to obtain a brown precipitate, and washed with water, and the resulting solid was re-dissolved in dichloromethane and washed with water , the solvent was removed under reduced pressure to give the crude product as a brown solid, which was triturated with methanol to afford intermediate one. This intermediate one structure is as follows:
[0048]
[0049] S2. Synthesis of intermediate 9-oxo-9H-thioxanthene-3,6-dicarboxylic acid:...
Embodiment 2
[0061] This example provides
[0062] Synthesis of N2,N6-bis(5-aminothiophen-2-yl)-9-oxo-9H-thioxanthene-2,6-dicarboxamide:
[0063]
[0064] S1. Synthesis of intermediate 9-oxo-9H-thioxanthene-2,6-dicarbonitrile:
[0065] Add 3.70g (0.01mol) of 2,6-dibromo-9H-thioxanthen-9-one, 4.478g (0.05mol) of cuprous cyanide, and 50ml of dry NMP into a 500ml three-necked flask, reflux at 140°C for 24h, and then Will H 2 O (180mL), HCl (60mL) and FeCl3 (4.19g, 25.8mmol) were poured into the reaction solution and stirred for 1h, cooled to room temperature, filtered to obtain a brown precipitate, and washed with water, and the resulting solid was re-dissolved in dichloromethane and washed with water , the solvent was removed under reduced pressure to give the crude product as a brown solid, which was triturated with methanol to afford intermediate one. This intermediate one structure is as follows:
[0066]
[0067] S2. Synthesis of intermediate 9-oxo-9H-thioxanthene-2,6-dicarboxy...
Embodiment 3
[0079] This example provides a synthetic
[0080] N2,N7-bis(7-aminodibenzo[b,d]furan-3-yl)-9-oxo-9H-thioxanthene-2,7-dicarboxami-de:
[0081]
[0082] S1. Synthesis of intermediate 9-oxo-9H-thioxanthene-2,7-dicarbonitrile:
[0083] Add 3.70g (0.01mol) of 2,7-dibromo-9H-thioxanthen-9-one, 4.478g (0.05mol) of cuprous cyanide, and 50ml of dry NMP into a 500ml three-necked flask, reflux at 140°C for 24h, and then Will H 2 O (180mL), HCl (60mL) and FeCl3 (4.19g, 25.8mmol) were poured into the reaction solution and stirred for 1h, cooled to room temperature, filtered to obtain a brown precipitate, and washed with water, and the resulting solid was re-dissolved in dichloromethane and washed with water , the solvent was removed under reduced pressure to give the crude product as a brown solid, which was triturated with methanol to afford intermediate one. This intermediate one structure is as follows:
[0084]
[0085]S2. Synthesis of intermediate 9-oxo-9H-thioxanthene-2,7-d...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com